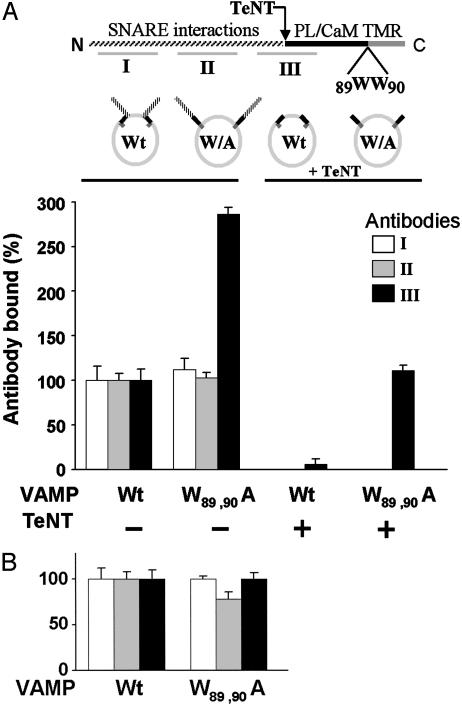
Fig. 2.
Cis lipid binding masks epitopes in the membrane-proximal domain of VAMP. Upper schema illustrates VAMP domains recognized by antibodies I, II, and III. The membrane-proximal region (black) is flanked N-terminally by the TeNT cleavage site (Q76–F77) and C-terminally by the transmembrane anchor (TMR). The mutation W89A, W90A inhibits lipid binding. PL, phospholipid; CaM, calmodulin. Lower schema represents the four VAMP liposome preparations. (A) Full-length recombinant wild type (Wt) or W89A, W90A mutant (W/A) VAMP was reconstituted into 25% DOPS/75% POPC liposomes (Left), and aliquots of each were treated with TeNT (Right). The four resulting liposome pools were immobilized on the sensor chip of an SPR apparatus and the binding of antibodies I (white bars), II (gray bars), and III (black bars) to each individual group was assayed. The data are presented as four groups of three bars. Bound antibody = mass (RU) of antibody bound/mass (RU) of VAMP liposome immobilized. Binding of each antibody to wild-type VAMP liposomes was normalized to 100%. Antibody III binding was corrected to account for direct effects of the W89A, W90A mutation (Fig. 6C). After TeNT treatment, binding of antibodies I and II was not detectable. Results are the means ± SD of three independent experiments, each in triplicate. (B) As in A Left, except that VAMP was reconstituted into 100% POPC. Results are the means ± SD of an experiment in quadruplicate.