Abstract
Background
Patients on dialysis have high rates of cardiovascular disease and are frequently treated with HMG-CoA reductase inhibitors. Given that these patients have insurance coverage for medications as well as regular contact with health care providers, differences by race in exposure to statins over time should be minimal among patients who are candidates for the drug.
Methods
We created a cohort of incident dialysis patients who were dually-eligible for Medicare and Medicaid services. We determined the proportion of days covered (or PDC, a marker of cumulative medication exposure) by a statin prescription over a mean of 2.0 ± 1.4 years. Ordinary least squares regression was used to determine the factors associated with cumulative drug exposure.
Results
Of the 18,727 patients who filled at least one prescription for a statin, mean PDC was 0.57 ± 0.32. The unadjusted PDC was higher for Caucasians (0.63 ± 0.31) than for African-Americans (0.51± 0.32), Hispanics (0.54 ± 0.31), and individuals of other race/ethnicity (0.58 ± 0.32). In multivariable modeling, Caucasian race was independently associated with greater exposure to statins. Relative to Caucasians, the adjusted odds ratios for the PDC for African-Americans was 0.47 (95% confidence intervals [CIs] 0.43 – 0.50), for Hispanics 0.52 (0.48 – 0.56) and for others, 0.72 (0.64 – 0.81).
Conclusions
Despite insurance coverage, regular contact with health care providers, and at least one prescription for a statin, there are large differences by race in statin exposure over time. The provider- and patient-associated factors related to this phenomenon should be further examined.
Keywords: HMG-CoA reductase inhibitors, statins, Medicare, Medicaid, medication exposure, cardiovascular disease, ESRD, dialysis
Introduction
Differences in care related to race and ethnicity have been abundantly demonstrated in the literature. For example, in the setting of cardiovascular disease care in the general population, minorities have lower rates of achieved cholesterol targets [1] and higher rates of hospital readmission [2] following cardiovascular events than do Caucasians. In the chronic dialysis environment, race-based differences in care have been noted in areas as diverse as placement of arteriovenous fistulas [3-5] and outcomes after renal transplantation [6, 7]. Understanding how care is impacted by race continues to be an important focus in kidney disease patients [8].
Patients on chronic dialysis have high rates of cardiovascular disease and are frequently treated with HMG-CoA reductase inhibitors (“statins”). We previously noted (in press) geographic variation in use of HMG CoA reductase inhibitors (“statins”) in a national U.S. sample of dialysis patients who were dually eligible for Medicare and Medicaid. Using a cross-sectional approach in which we ascertained whether any statin exposure occurred, we found in unadjusted analyses that African-Americans had a relative decrease in statin use of >25% compared to Caucasians; use rates were lower for Hispanics as well. Even after adjustment for a broad range of other factors, this finding remained statistically significant, with African-Americans still 23% less likely to have received a statin than their Caucasian peers.
In the present report, we extend our investigation of statin exposure in chronic dialysis patients by examining the association of race with long-term statin exposure. Although race-based differences in cumulative medication exposure should be less likely in the dialysis environment, a setting in which all patients have similarly frequent contact with health care providers, differences in medication use by race have been shown to exist even in environments in which there “universal” access to care (such as in the Veterans Administration system [9, 10] or in state Medicaid programs [11]).
To investigate whether statin use over time varies by race in dialysis patients, we utilized a novel linkage of Medicare claims data and state Medicaid prescription claims [12]. Although dually-eligible patients, who represent approximately 32% of prevalent dialysis patients, differ from the general dialysis population in that they are more likely to be younger, female, and of minority-race [13], they nevertheless represent a uniquely informative population of study because their Medicare and Medicaid data can be linked without requiring access to proprietary sources, and because their medication exposure over time can be reliably tracked and. Although the results of recent statin trials in dialysis patients have been mixed [14-16], a state of clinical equipoise about the utility of the drugs existed during our period of study. Since there was no basis for supposing that the drugs’ benefits would vary by race, statin exposure patterns are therefore a useful tool by which one can examine the broader issue of how cumulative exposure to drugs might vary by race. We modeled only individuals who received at least one prescription for a statin, thereby confining our analysis to individuals who were judged suitable candidates for the drug. If, after adjusting for other key factors, race is associated with differential exposure to statins, future work examining the reasons behind race-based differences in medication exposure could be undertaken.
Methods
Data Sources for Analysis
We identified a cohort of dually-eligible (Medicare-Medicaid) patients initiating chronic dialysis from 2000-2005. As previously described [12], we were able to link data from the United States Renal Data System (USRDS) including the Core CD and Medicare Part A and Part B claims with Medicaid Analytic eXtract_ENREF_20 Personal Summary Files and prescription drug claims files.
Study Cohort and Rationale for Analytic Approach
We analyzed individuals over the age of 18 years who survived at least 90 days after dialysis initiation and who were continuously enrolled in Medicare and Medicaid from dialysis initiation until the end of the observation period during. After surviving the initial 90-day survival run-in period (in accordance with USRDS guidelines), individuals were censored when they lost either Medicare or Medicaid eligibility, received a kidney transplant, died, or reached the end of the observation window (12/31/2005). Patients enrolled in Medicaid managed care plans (e.g., as in Arizona or Tennessee) or in the Department of Veterans Affairs health system were excluded since medication data were not available. (Of note, persons on chronic dialysis were not generally eligible for Medicare managed care plans prior to 2006.) We excluded residents of Ohio since the days of medication supplied was not recorded in the claim. To assure that eligible patients were actually accessing benefits, we eliminated individuals who did not fill at least one Medicaid prescription during the first 90 days of dialysis. Finally, since our goal was study outpatient medication use over time, we eliminated individuals who were institutionalized ≥ 33% of their respective observation windows, as inpatient medication exposure is not observable.
Descriptive Variables
Demographic and clinical variables were drawn from the CMS 2728 dialysis intake form. Demographic variables included age, sex, and race by ethnicity (four mutually-exclusive groups comprising non-Hispanic Caucasians, non-Hispanic African-Americans, Hispanics, and Others), and employment status. Risk behaviors included smoking and substance abuse (alcohol or illicit drugs), and functional status markers examined were the ability to ambulate and to transfer. Cause of ESRD was categorized as diabetes, hypertension, glomerulonephritis, or other. Major clinical comorbidities were diabetes, congestive heart failure, coronary artery disease, cerebrovascular disease, and peripheral vascular disease. We supplemented the medical information from the CMS 2728 form with an adapted version of the Liu comorbidity index [17], a summary measure of comorbidity burden; we used only 90 (rather than Liu et al's original 180) days in which to acquire claims since we found little difference in indices generated using 90 or 180 days of claims data. Since the CMS 2728 form is structured such that diseases like diabetes or hypertension may be considered as both a cause of ESRD and/or a comorbidity, diabetes and hypertension were considered present in an individual if they were listed as either the cause of ESRD or as a comorbidity. Baseline hemoglobin was dichotomized at 11g/dL. Dialysis modality at time of dialysis initiation was categorized as in-center hemodialysis (HD) or self-care dialysis (home HD or peritoneal dialysis (PD)).
Medication Exposure
We matched drug name and therapeutic class information for statins in the Medicaid drug claims at the national drug code (NDC) level using Multum Lexicon (Cerner Corporation, www.cerner.com). Details of our specific medication identification strategy are described elsewhere [12]. Individuals were characterized as statin users if they received at least one prescription for a statin during their respective observation windows. We determined the days supplied from the Medicaid prescription claims files to establish the proportion of days covered, or PDC [18, 19]. If a subsequent prescription was filled more than 7 days before the completion of the previous prescription, therapy was considered “changed”; that is, the previous prescription was truncated and superseded by the ensuing prescription. If a subsequent prescription was filled ≤ 7 days prior to the completion of the previous prescription, the overlap was “carried forward”; that is, exposure to the ensuing prescription was lengthened by the duration of the overlap. For time spent in the hospital, we made no assumptions regarding medication exposure; thus, those days were excluded from calculation of the medication exposure altogether. Each patient's medication exposure was then calculated as his or her proportion of non-hospitalized days observed.
Statistical Analyses
We generated descriptive statistics (means and standard deviations for continuous variables and frequencies for categorical variables) to examine the distributions of our study measures for statin use. Percentiles (median, 25th, and 75th) were also generated for the medication exposure measures. Bivariate analyses comparing each of the explanatory variables by use versus non-use were performed using Pearson's chi-squared test or Student's t-test, as appropriate (data not shown). To identify independent factors associated with statin use specifically among those who received at least one statin prescription, we generated ordinary least squares (OLS) regression models with medication status being regressed simultaneously on all a priori selected explanatory variables. Each model was limited to users of that medication (i.e., non-users were excluded). The response measures ranged from (0, 1], and residual analyses indicated failure to adhere to OLS regression model assumptions (not shown). Thus, we transformed our response measures using the logit transformation. (For subjects with a medication exposure value equal to one, we added a single day to their denominator to produce a value less than one as the logit transformation of one does not exist due to division by zero.) The use of the logit transformation enabled parameter interpretation in the familiar context of adjusted odds ratios (AORs). To account for the different between-subject follow-up times for the response measure, we weighted the OLS regression models by the number of days in the cohort for each subject. Visual inspection of residual plots and their empiric histograms were used for model assessment.
Due to the large sample size, statistical significance was inferred only when P < 0.01. All statistical analyses were done with SAS 9.2 (SAS Institute, Inc., www.sas.com).
Compliance and Research Participant Protection
The research protocol was approved by the institutional review board at the University of Kansas Medical Center (KUMC), and the project was undertaken according to the principles of the Declarations of Helsinki. Data Use Agreements were in place between KUMC and the USRDS and CMS.
Results
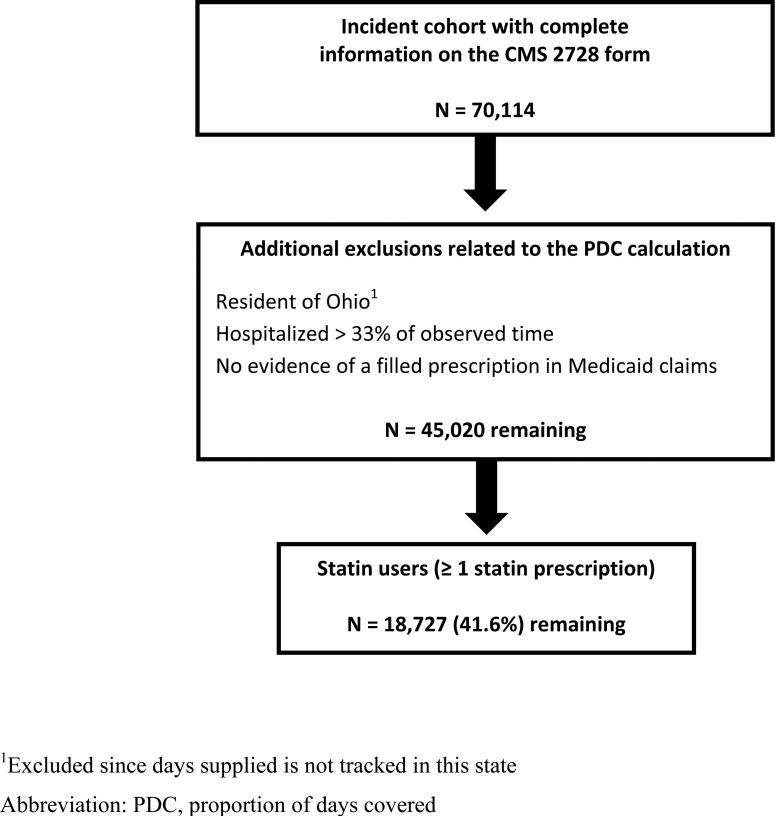
A total of 70,114 dialysis patients initiating dialysis between January 1, 2000 and December 31, 2005 met the initial inclusion criteria (dual eligibility, age ≥ 18 years, single state of residence, survival > 90 days after dialysis initiation, and no Department of Veterans Affairs coverage). As shown in Figure 1, the cohort was then further refined by excluding residents of Ohio (as no record of days of medication supplied is kept by Medicaid in this state), individuals who were hospitalized > 33% of their observation window, and those who, despite having Medicaid benefits, were not accessing pharmacy benefits them; this resulted in 45,020 individuals remaining. Users of statins numbered 18,727 (41.6%).
Figure 1.
Flow diagram for the construction of the study cohort.
The characteristics of the statin users versus the non-users are shown in Table 1. Stain users were significantly more likely to be older, female, and Caucasian, have better functional status, have a tendency toward higher BMIs, and have higher rates of each of the assessed comorbidities (also reflected in a higher overall Liu comorbidity score). Only about 1 in 20 in each of the groups engaged in self-care dialysis. Mean observation time was 2.0 ± 1.4 years in the statin users and 1.6 ± 1.3 years in the non-users.
Table 1.
Descriptive statistics for dually-eligible dialysis patients using statins.
| Characteristic | Users 18,727 (41.6%) | Nonusers 26,293 (58.4%) | P-value |
|---|---|---|---|
| Age, years | 62.1 ± 13.4 | 59.5 ± 16.8 | < 0.0001 |
| Female gender, % | 61.6 | 53.3 | < 0.0001 |
| Race/ethnicity, % | < 0.0001 | ||
| African-American | 35.6 | 42.6 | |
| Caucasian | 34.9 | 32.9 | |
| Hispanic | 21.6 | 18.3 | |
| Other | 7.9 | 6.3 | |
| Unable to ambulate, % | 4.2 | 5.2 | < 0.0001 |
| Unable to transfer, % | 1.2 | 1.8 | < 0.0001 |
| Unemployed, % | 98.2 | 97.1 | < 0.0001 |
| BMI category, % | < 0.0001 | ||
| < 20 kg/m2 | 7.0 | 12.4 | |
| 20 – < 25kg/m2 | 26.6 | 32.4 | |
| 25 – < 30 kg/m2 | 28.3 | 25.9 | |
| ≥ 30 kg/m2 | 38.2 | 29.3 | |
| Smoker, % | 5.5 | 7.4 | < 0.0001 |
| Substance abuser, % | 1.4 | 4.0 | < 0.0001 |
| Cause of ESRD, % | < 0.0001 | ||
| Diabetes | 64.8 | 44.6 | |
| Hypertension | 20.9 | 29.3 | |
| Glomerulonephritis | 5.5 | 9.6 | |
| Other | 8.8 | 16.5 | |
| Adapted Liu comorbidity score | 6.7 ± 3.4 | 5.8 ± 3.6 | < 0.0001 |
| Adapted Liu distribution, %: | < 0.0001 | ||
| 0 | 2.8 | 6.4 | |
| 1-5 | 38.4 | 45.7 | |
| 6-10 | 45.1 | 36.5 | |
| 11+ | 13.8 | 11.4 | |
| Major comorbidities, % | |||
| DM | 74.8 | 53.7 | < 0.0001 |
| HTN | 86.6 | 84.3 | < 0.0001 |
| CHF | 35.3 | 30.6 | < 0.0001 |
| CAD | 30.2 | 19.2 | < 0.0001 |
| PVD | 15.9 | 12.1 | < 0.0001 |
| CVA | 11.2 | 9.2 | < 0.0001 |
| Hb at baseline < 11.0 g/dL, % | 72.6 | 75.9 | < 0.0001 |
| In-center hemodialysis, % | 93.5 | 95.7 | < 0.0001 |
| Years in the cohort | 2.0 ±1.4 | 1.6 ± 1.3 | < 0.0001 |
“Users” refers to users of HMG CoA-reductase inhibitors, or statins
Abbreviations: BMI, body mass index; ESRD, end stage renal disease; HTN, hypertension; DM, diabetes; CHF, congestive heart failure; CAD, coronary artery disease; PVD; peripheral vascular disease; CVA, cerebrovascular accident; Hb, hemoglobin
The unadjusted PDCs for statins, restricted to individuals who received at least one statin prescription, are shown by race in Table 2. For the overall study sample, the PDC was 0.57 ± 0.32, with a 25% percentile of 0.26, a 50% percentile of 0.61, and a 75% percentile of 0.87; that medians (50th percentiles) were similar to means suggests there was minimal skew to the distribution. However, PDCs varied by race: the PDC was 0.63 ± 0.31 for Caucasians, 0.51 ± 0.32 for African-Americans, 0.54 ± 0.31 for Hispanics, and 0.58 ± 0.32 for others.
Table 2.
Unadjusted distribution of proportion of days covered, by race.
| Racial Group | Mean ± SD | 25th% | 50th% | 75th% |
|---|---|---|---|---|
| Overall | 0.57 ± 0.32 | 0.26 | 0.61 | 0.87 |
| Caucasians | 0.63 ± 0.31 | 0.36 | 0.72 | 0.91 |
| African-Americans | 0.51 ± 0.32 | 0.21 | 0.52 | 0.81 |
| Hispanics | 0.54 ± 0.31 | 0.25 | 0.58 | 0.83 |
| Other | 0.58 ± 0.32 | 0.28 | 0.65 | 0.89 |
SD, standard deviation
In the ordinary least squares model of the relationship between modeled characteristics and cumulative statin use, several findings were significant (Table 3). Increasing age, BMI ≥ 30kg/m2, coronary artery disease, and history of a cerebrovascular accident were all significantly associated with increased exposure (P < 0.0001 in each case); peripheral vascular disease was not.
Table 3.
Ordinary least squares regression models for the adjusted odds ratios of factors associated with statin exposure over time, measured as the proportion of days covered.
| Characteristic | AOR | 95% CIs | P-value |
|---|---|---|---|
| Age, per 10 yrs | 1.23 | 1.20 – 1.26 | < 0.0001 |
| Male sex | 1.02 | 0.96 – 1.09 | 0.44 |
| Race/Ethnicity | |||
| Caucasian | – | – | – |
| African-American | 0.47 | 0.43 – 0.50 | < 0.0001 |
| Hispanic | 0.52 | 0.48 – 0.56 | < 0.0001 |
| Other | 0.72 | 0.64 – 0.81 | < 0.0001 |
| Inability to ambulate | 1.10 | 0.93 – 1.30 | 0.27 |
| Inability to transfer | 0.87 | 0.63 – 1.20 | 0.39 |
| Employed | 1.23 | 0.99 – 1.53 | 0.067 |
| BMI category | |||
| < 20 kg/m2 | 0.88 | 0.78 – 0.99 | 0.05 |
| 20-24.9 kg/m2 | – | – | – |
| 25-29.9 kg/m2 | 1.11 | 1.03 – 1.20 | 0.0080 |
| 30+ kg/m2 | 1.19 | 1.10 – 1.28 | < 0.0001 |
| Smoker | 0.84 | 0.74 – 0.96 | 0.0082 |
| Substance abuser | 0.84 | 0.65 – 1.07 | 0.15 |
| Liu Comorbidity Score2 | 1.11 | 1.04 – 1.18 | 0.0029 |
| Comorbidities | |||
| DM | 1.31 | 1.22 – 1.41 | < 0.0001 |
| HTN | 1.07 | 0.98 – 1.16 | 0.11 |
| CHF | 0.94 | 0.87 – 1.01 | 0.10 |
| CAD | 1.19 | 1.10 – 1.27 | < 0.0001 |
| PVD | 0.98 | 0.89 – 1.07 | 0.62 |
| CVA | 1.26 | 1.15 – 1.39 | < 0.0001 |
| Hb < 11.0 | 0.81 | 0.76 – 0.86 | < 0.0001 |
| Self-care dialysis | 1.12 | 1.00 – 1.26 | 1.12 |
Abbreviations: AOR, adjusted odds ratios; BMI, body mass index; HTN, hypertension; DM, diabetes; CHF, congestive heart failure; CAD, coronary artery disease; PVD; peripheral vascular disease; CVA, cerebrovascular accident; Hb, hemoglobin.
Race had a marked independent effect on statin exposure. After adjustment for all other factors, African-Americans had an AOR for statins of 0.47 relative to Caucasians (95% confidence intervals [CIs] 0.43 – 0.50), Hispanics 0.52 (0.48 – 0.56), and individuals of other race/ethnicity 0.72 (0.64 – 0.81), with P < 0.0001 in each case.
Discussion
In this study, we applied a measure of cumulative drug exposure to a large cohort of incident dialysis patients to determine the factors associated with statin use over time. Our most salient finding is that race/ethnicity was strongly associated with cumulative statin exposure. Since we deliberately restricted our analysis to individuals who had at least one prescription for a statin, all studied individuals were deemed suitable candidates for these drugs at some point by their healthcare providers. Nevertheless, the odds of cumulative exposure of statins in minorities was, collectively, only slightly more than half that of Caucasians.
There is a paucity of work examining the association between race/ethnicity and medication exposure in dialysis patients. A small study of patient–related adherence to antihypertensive medications in hemodialysis patients reported that adherence was lower for minorities [20], suggesting the possibility that exposure over time in minorities could be less than for non-minorities. To investigate this issue in depth, we employed a more rigorous approach than any used previously, specifically one which utilized the fine detail provided by a PDC-based approach to drug exposure over time. To our knowledge it has not been used in the dialysis population by any other group.
Why minorities appear to have less overall exposure to statins is uncertain, since factors contributing to differences in care are complex and encompass both patient-centric factors (e.g., health literacy or healthcare costs [21]) as well as provider-centric ones (e.g., provider behavior or site of care [22]). One possibility is that medications might lapse, only to be restarted at a later date, at higher rates in non-Caucasians than in Caucasians, the result being that minorities might have more discontinuous use of statins and other medications. It could also be the case that differences among provider beliefs concerning the relative risks and benefits of such agents across racial groups could be responsible for this phenomenon, or that adherence over time in filling or refilling prescriptions is lower in non-Caucasians than in Caucasians. However, it would be incorrect to describe our results as measuring medication adherence per se, since it is impossible to determine with the present data whether either patients or providers are making overt or conscious decisions to cease or modify therapy.
Few studies have formally examined factors associated with statin prescribing. Using the international cohort from DOPPS (Dialysis Outcomes and Practice Patterns Study) I, Mason et al [23] found associations of coronary artery disease and diabetes with statin prescription at study entry, echoing our findings. However, they reported an association between PVD and statin use, a finding we did not reproduce. These investigators did not model a history of CVAs, nor did they include information on BMI. That we found these latter factors to be associated with statin use has an intuitive appeal: individuals who have a CVA might be expected to be more aggressively screened and treated for hyperlipdiemia than others, while individuals with a high BMI (which is a marker for nutritional status in dialysis patients) would be more likely to have high cholesterol levels than the presumably frailer or sicker individuals with lower BMIs. Why each of these factors appears to be associated with statin use should be the subject of future detailed study.
It is also important to note that it remains uncertain whether statins actually confer any benefit to chronic dialysis patients, so it would be premature to label differential use of medications by race as a “disparity.” Thus we used longitudinal statin prescriptions merely as a window through which we could examine how patterns of care vary over time by race.
Our study has several important limitations. First, we studied only dually-eligible patients, who represent the neediest patients, so caution should be used when attempting to generalize to the general dialysis population. Since the dually-eligible patient population has higher rates of younger individuals, women, and minorities, our results may not reflect chronic dialysis population as a whole. Second, we used pharmacy prescription records to infer medication exposure. This does not equate to proof of medication use, but it seems unlikely that patients would fill prescriptions repeatedly for medications which they are not ingesting. Third, some patients may have qualified for Medicaid after they met a certain threshold of out-of-pocket costs before qualifying for full coverage (a phenomenon known as “spend-down”); to minimize this bias we required that all patients demonstrate actual use of the Medicaid prescription drug benefit during the initial 90 days of dialysis.
In summary, in a cohort of dually-eligible chronic dialysis patients wherein vascular risk is high, cumulative exposure to statins was substantially lower in non-Caucasians relative to Caucasians. The underlying factors responsible for this finding are uncertain at present, so further study is warranted.
Acknowledgements
The authors thank Connie Wang, MD, for technical assistance in manuscript preparation.
Sources of Funding: Funding for this study was provided by NIH (NIDDK) grants R01 DK080111-02 (T.I.S.) and K23 DK085378-01 (J.B.W.), a National Kidney Foundation Young Investigator Award (J.B.W.), and a Sandra A. Daugherty Foundation Grant (J.B.W.).
Footnotes
Disclosures: The authors have no conflicts of interest to declare
Disclaimer: The data reported here have been supplied by the United States Renal Data System (DUA#2007-10 & 2009-19) and the Centers for Medicare & Medicaid Services (DUA#19707). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government.
References
- 1.Trivedi AN, Zaslavsky AM, Schneider EC, Ayanian JZ. Relationship between quality of care and racial disparities in Medicare health plans. Jama. 2006;296:1998–2004. doi: 10.1001/jama.296.16.1998. [DOI] [PubMed] [Google Scholar]
- 2.Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. Jama. 2011;305:675–681. doi: 10.1001/jama.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wasse H, Speckman RA, Frankenfield DL, Rocco MV, McClellan WM. Predictors of delayed transition from central venous catheter use to permanent vascular access among ESRD patients. Am J Kidney Dis. 2007;49:276–283. doi: 10.1053/j.ajkd.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hopson S, Frankenfield D, Rocco M, McClellan W. Variability in reasons for hemodialysis catheter use by race, sex, and geography: findings from the ESRD Clinical Performance Measures Project. Am J Kidney Dis. 2008;52:753–760. doi: 10.1053/j.ajkd.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Cavanaugh KL, Wingard RL, Hakim RM, Elasy TA, Ikizler TA. Patient dialysis knowledge is associated with permanent arteriovenous access use in chronic hemodialysis. Clin J Am Soc Nephrol. 2009;4:950–956. doi: 10.2215/CJN.04580908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakkera HA, O'Hare AM, Johansen KL, Hynes D, Stroupe K, Colin PM, Chertow GM. Influence of race on kidney transplant outcomes within and outside the Department of Veterans Affairs. J Am Soc Nephrol. 2005;16:269–277. doi: 10.1681/ASN.2004040333. [DOI] [PubMed] [Google Scholar]
- 7.Hall YN, Choi AI, Xu P, O'Hare AM, Chertow GM. Racial ethnic differences in rates and determinants of deceased donor kidney transplantation. J Am Soc Nephrol. 2011;22:743–751. doi: 10.1681/ASN.2010080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powe NR. Let's get serious about racial and ethnic disparities. J Am Soc Nephrol. 2008;19:1271–1275. doi: 10.1681/ASN.2008040358. [DOI] [PubMed] [Google Scholar]
- 9.Poon I, Lal LS, Ford ME, Braun UK. Racial/ethnic disparities in medication use among veterans with hypertension and dementia: a national cohort study. Ann Pharmacother. 2009;43:185–193. doi: 10.1345/aph.1L368. [DOI] [PubMed] [Google Scholar]
- 10.Mehta JL, Bursac Z, Mehta P, Bansal D, Fink L, Marsh J, Sukhija R, Sachdeva R. Racial disparities in prescriptions for cardioprotective drugs and cardiac outcomes in Veterans Affairs Hospitals. Am J Cardiol. 2010;105:1019–1023. doi: 10.1016/j.amjcard.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 11.Litaker D, Koroukian S, Frolkis JP, Aron DC. Disparities among the disadvantaged: variation in lipid management in the Ohio Medicaid program. Prev Med. 2006;42:313–315. doi: 10.1016/j.ypmed.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Wetmore JB, Mahnken JD, Mukhopadhyay P, Hou Q, Ellerbeck EF, Rigler SK, Spertus JA, Shireman TI. Geographic variation in cardioprotective antihypertensive medication usage in dialysis patients. Am J Kidney Dis. 58:73–83. doi: 10.1053/j.ajkd.2011.02.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wetmore JB, Rigler SK, Mahnken JD, Mukhopadhyay P, Shireman TI. Considering health insurance: how do dialysis initiates with Medicaid coverage differ from persons without Medicaid coverage? Nephrol Dial Transplant. 2010;25:198–205. doi: 10.1093/ndt/gfp396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 15.Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Gronhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Suleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wuthrich RP, Gottlow M, Johnsson E, Zannad F. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 16.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellstrom B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Gronhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int. 2010;77:141–151. doi: 10.1038/ki.2009.413. [DOI] [PubMed] [Google Scholar]
- 18.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. Jama. 2002;288:455–461. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 19.Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009;15:728–740. doi: 10.18553/jmcp.2009.15.9.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtin RB, Svarstad BL, Keller TH. Hemodialysis patients’ noncompliance with oral medications. Anna J. 1999;26:307–316. discussion 317, 335. [PubMed] [Google Scholar]
- 21.Charles H, Good CB, Hanusa BH, Chang CC, Whittle J. Racial differences in adherence to cardiac medications. J Natl Med Assoc. 2003;95:17–27. [PMC free article] [PubMed] [Google Scholar]
- 22.Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, Ho PM, Spertus JA. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH): design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–476. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mason NA, Bailie GR, Satayathum S, Bragg-Gresham JL, Akiba T, Akizawa T, Combe C, Rayner HC, Saito A, Gillespie BW, Young EW. HMG-coenzyme a reductase inhibitor use is associated with mortality reduction in hemodialysis patients. Am J Kidney Dis. 2005;45:119–126. doi: 10.1053/j.ajkd.2004.09.025. [DOI] [PubMed] [Google Scholar]