FIGURE 1:
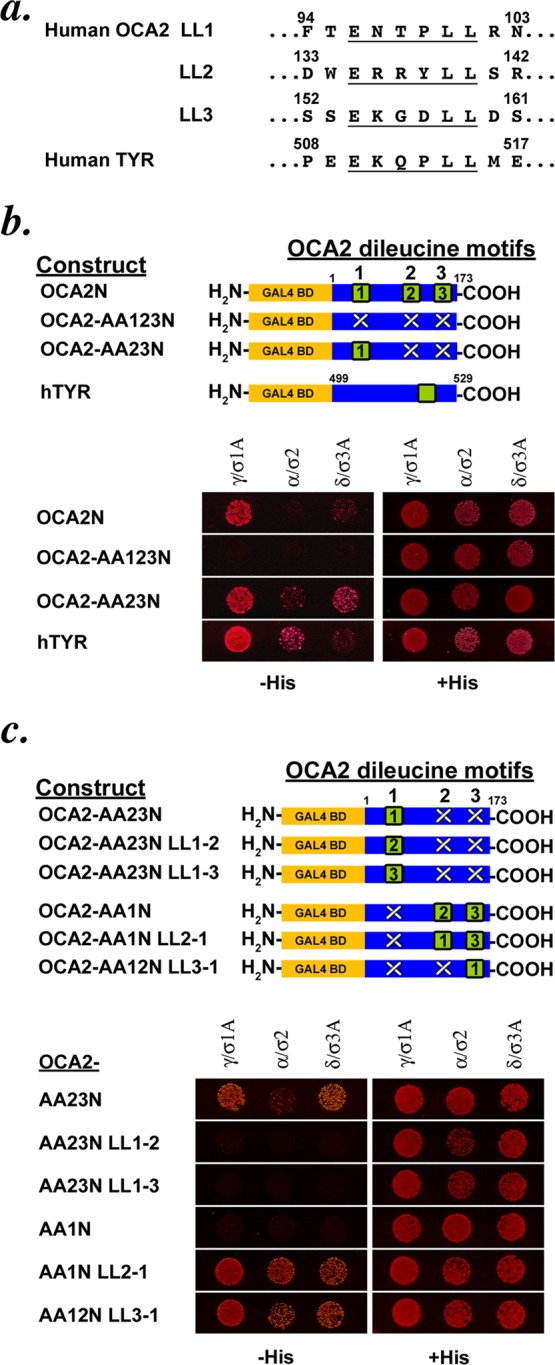
The cytoplasmic domain of OCA2 shows dileucine-dependent interaction with adaptor proteins in a yeast three-hybrid system. (a) Sequences of the three acidic dileucine sorting motifs in the cytoplasmic N-terminus of human OCA2 and the single acidic dileucine motif in the cytoplasmic C-terminus of human tyrosinase. (b) Schematic of OCA2 N-terminal-domain Gal4 fusion constructs used in the assay. GAL4 BD, Gal4-binding domain; green square, intact dileucine motif; white X, disrupted dileucine motif. Black numbers within the green square indicate whether the primary sequence of the LL1, LL2, or LL3 motif is inserted at the indicated position within the domain. Below, a yeast three-hybrid assay of various OCA2 constructs coexpressed with hemicomplexes of the AP-1 (γ/σ1A), AP-2 (α/σ2), and AP-3 (δ/σ3A) complexes. A protein–protein interaction leads to expression of HIS3, allowing growth on His-deficient medium. All transformed yeast grow on His-containing control medium. (c) Schematic of OCA2 constructs used in the assay. Below, yeast three-hybrid assay of the OCA2 constructs in which dileucine motif sequences are repositioned within the OCA2 cytoplasmic domain.
