FIGURE 1:
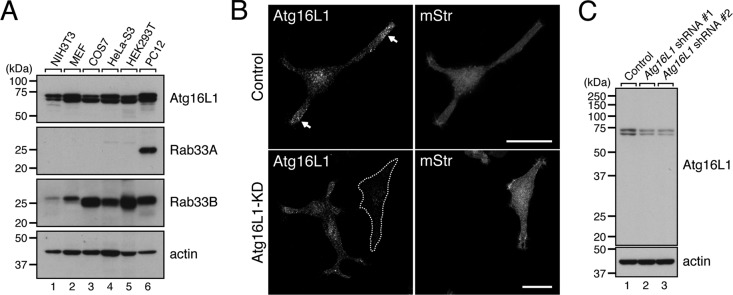
Endogenous Atg16L1 protein in PC12 cells is localized in the neurites. (A) Total lysates of various cell lines indicated were subjected to 10% SDS–PAGE followed by immunoblotting with anti-Atg16L1 antibody (top), anti-Rab33A antibody (second from top), anti-Rab33B antibody (third from top), and anti-actin antibody (bottom). Note that expression of Rab33A was restricted to PC12 cells (lane 6), whereas both Atg16L1 and Rab33B were expressed in all cell lines tested. (B) Unique localization of Atg16L1 in the neurites of PC12 cells. PC12 cells were cotransfected with Atg16L1 shRNA (or control shRNA) and pmStr-C1 vector as a transfection marker and then immunostained with a specific antibody against Atg16L1 (see C). Note that the Atg16L1 signals were strongly concentrated in the neurites (top left, arrows) and that these signals did not appear after knockdown (KD) of Atg16L1 with Atg16L1 shRNA #1 (an Atg16L1-KD cell is outlined in a broken line; bottom). Scale bars, 20 μm. (C) Specificity of anti-Atg16L1 antibody as revealed by immunoblotting. The anti-Atg16L1 antibody specifically recognized doublet bands of Atg16L1 (lane 1), which correspond to the α and β forms of Atg16L1 (Mizushima et al., 2003; Ishibashi et al., 2011), and these signals were clearly attenuated by treatment with two independent Atg16L1 shRNAs (lanes 2 and 3). PC12 cells were transfected with Atg16L1 shRNA #1 or Atg16L1 shRNA #2 and then cultured for 5 d. The cells were solubilized with 1% Triton X-100, and their lysates were subjected to 10% SDS–PAGE followed by immunoblotting with anti-Atg16L1 antibody (top) and anti-actin antibody (bottom). The size of the molecular mass markers (in kDa) is shown at the left.