Synopsis
Objectives
The aim of the present study was to assess fluconazole pharmacokinetic measures in serum and cerebrospinal fluid (CSF); and the correlation of these measures with clinical outcomes of invasive fungal infections.
Methods
A randomized trial was conducted in HIV-infected patients receiving 3 different regimens of fluconazole plus amphotericin B (AmB) for the treatment of cryptococcal meningitis. Regimens included fluconazole 400 mg/day+AmB (AmB+Fluc400) or fluconazole 800 mg/day+AmB (AmB+Fluc800) (14 days followed by fluconazole alone at the randomized dose for 56 days); or AmB alone for 14 days followed by fluconazole 400 mg/day for 56 days. Serum (at 24 hours after dosing) and CSF samples were taken at Baseline and days 14 and 70 (serum only) for fluconazole measurement, using gas-liquid chromatography.
Results
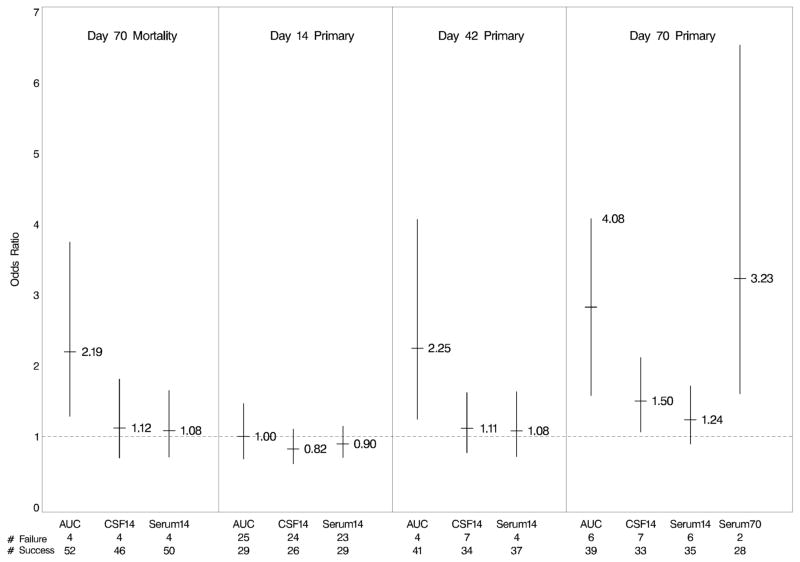
Sixty-four treated patients had fluconazole measurements; 11 in AmB group, 12 in AmB+Fluc400 group and 41 in AmB+Fluc800 group. Day 14 serum concentration geometric means were 24.7 mg/L for AmB+Fluc400 and 37.0 mg/L for AmB+Fluc800. Correspondingly, CSF concentration geometric means were 25.1 mg/L and 32.7 mg/L. Day 14 Serum and CSF concentrations were highly correlated for AmB+Fluc800 (p<0.001, r=0.873) and for AmB+Fluc400 (p=0.005, r=0.943). Increased Serum AUC appears associated with decreased mortality at day 70 (p=0.061, odds-ratio=2.19) as well as with increased study composite endpoint success at Days 42 and 70 (p=0.081, odds-ratio=2.25 and 0.058, 4.08; respectively).
Conclusion
High fluconazole dosage (800 mg/day) for the treatment of HIV-associated cryptococcal meningitis was associated with high serum and CSF fluconazole concentration. Overall, high serum and CSF concentration appear associated with increased survival and primary composite endpoint success.
Introduction
Cryptococcus neoformans can cause significant morbidity and mortality in the immuno-compromised host, and invasion of the central nervous system (CNS) may lead to devastating consequences.1 Fluconazole is a triazole antifungal agent that has a long half-life and excellent bioavailability, exhibits low serum protein binding, and achieves high levels in multiple tissues, including the CNS.2 This medication is excreted unchanged in the urine; hepatic CYP2C9 enzyme plays a minor role.2 Treatment of CNS infections is frequently difficult because the blood brain barrier limits diffusion of drug into the CNS; however, the ability of fluconazole to penetrate CSF increases during meningeal inflammation. Furthermore, tissue efflux pumps can reduce CNS drug accumulation.3 To date, data regarding the relationship between the pharmacokinetics of fluconazole in serum and cerebrospinal fluid (CSF); and in the correlation of these pharmacokinetic measures with clinical outcomes of invasive fungal infections in humans are limited.4
BAMSG 3-01 was a Phase II, multicenter, randomized clinical trial to investigate the safety and efficacy of combination therapy of amphotericin B plus fluconazole for treatment of HIV-associated cryptococcal meningitis.5 A secondary objective was to assess fluconazole pharmacokinetics and pharmacodynamics by (1) examining the relationship between serum and CSF concentrations in subjects receiving high-dose fluconazole, (2) identifying baseline characteristics influencing serum and CSF concentrations and (3) determining the relationship of serum and CSF drug concentrations with fluconazole dosing, efficacy measures, and post-baseline characteristics of interest.
Materials and methods
Standard therapy consisted of amphotericin B (AmB) (0.7 mg/kg/day) for 14 days followed by 56 days of oral fluconazole (400 mg/day). The 2 combination therapy arms consisted of the same dosage of AmB combined with either once daily 400 mg or 800 mg of oral fluconazole beginning at baseline (AmB+Fluc400 and AmB+Fluc800 respectively). Randomization was stratified by baseline opening CSF pressure (≤250 vs. >250 mmCSF vs. “not done”) and country (United States vs. Thailand).
Serum and CSF samples were taken at baseline, day 14, at highly-active antiretroviral treatment (HAART) initiation (serum only), and day 70 (end of treatment; serum only) from all 41 subjects receiving AmB+Fluc800 and the first 11 subjects receiving AmB+Fluc400 and first 12 receiving AmB. Specifically, 64 subjects had a serum sample at baseline, 54 at day 14, 22 at HAART initiation, and 30 at day 70 and 51 subjects had a CSF sample at baseline and 50 at day 14. All 64 subjects included in the analyses had a mortality outcome; however, 3 subjects did not have the Day 14 primary outcome and 12 did not have a Day 42 or Day 70 primary outcome.
Serum was obtained at 24 hours after dosing. Samples were analyzed using gas-liquid chromatography.6 The extraction recovery rate was approximately 85–90%, and the resulting assay was linear from 0.2–200 mg/L (lower limit of detection=0.2). Area under the curve from start of study to last assessment (AUC0-last) was calculated for the fluconazole serum concentration using actual assessment dates and the linear trapezoidal rule. Since the length of follow-up time varied across subjects, the weighted mean AUC0-last was calculated as the AUC normalized by actual days of follow-up (AUCSerum). Since there were few serum samples per subject (~4), this measure is considered an approximation of mean daily concentration.
Analyses included all subjects receiving at least 1 dose of study therapy with at least 1 post-baseline CSF or serum sample. Because BAMSG 3-01 was not powered to formally assess fluconazole concentration, p-values presented are descriptive and do not represent formal hypothesis tests and no adjustments were made for multiple testing. The following pharmacokinetic measures were summarized by treatment arm: day 14 serum (CSerum14 ) and CSF concentration (CCSF14), day 70 serum concentration (CSerum70), and AUCSerum. To examine the relationship between CSerum14 and CCSF14, the Pearson correlation coefficient was calculated. To identify baseline characteristics impacting concentration, linear regression models adjusting for dose received (through day 14 for CSerum14 and CCSF14 and through day 70 for CSerum70 and AUCSerum) using each natural log-transformed pharmacokinetic measure as the outcome were created for each characteristic. For each pharmacokinetic measure, any characteristics with p-value ≤0.20 for this univariate association with the pharmacokinetic measure were included in a multivariable model (final model obtained using backwards selection; characteristics retained in final model if p-value ≤0.10). Baseline characteristics explored include: country, age, body mass index (BMI), weight, serum creatinine, creatinine clearance (CrCl), eGFR, HAART status, CSF opening pressure, CSF WBC, CSF protein, CSF cryptococcal antigen titer, viral load, and CD4+T-cell count. Linear regression models were also used to assess the relationship of each natural log-transformed pharmacokinetic measure and dose received and the impact of concentration on post-baseline characteristics of interest (serum creatinine, CrCl, eGFR, HAART status, CSF opening pressure, CSF WBC, CSF Protein, and CSF cryptococcal antigen titer). Logistic regression models were used to assess the association between each clinical endpoint (day 70 morality status and day 14, day 42 and 70 study composite endpoint statuses [success defined as culture-negative, alive, and neurologically stable]) and the natural log-transformed pharmacokinetic measures. This clinical trial is registered in the National Library of Medicine’s registry (www.clinicaltrials.gov) under the registration number NCT00145249.
Results
Table 1 summarizes fluconazole pharmacokinetic parameters by treatment arm and Table 2 displays the association between pharmacokinetic parameters and subject characteristics. Numerically, the geometric mean CSerum14 for AmB+Fluc800 was greater than AmB+Fluc400. The same trend was seen for CSerum70 and CCSF14. Additionally, CSerum14 and CCSF14 were highly correlated for AmB+Fluc800 (p<0.001, r=0.873) and for AmB+Fluc400 (p=0.005, r=0.943). Decreased estimated glomerular filtration (eGFR), decreased viral load, and no HAART at baseline were associated with increased pharmacokinetic concentration. In the model for AUCSerum, there was a significant interaction between fluconazole dose and eGFR; as dose received increased, the impact of eGFR decreased. With respect to post-baseline characteristics, high pharmacokinetic concentration was associated with low CSF WBC count and decreased renal function.
Table 1.
Summary of pharmacokinetic parameters
| AmB+Fluc400 | AmB+Fluc800 | p-valuea | |
|---|---|---|---|
| CCSF 14 (mg/L) | |||
| n | 9 | 33 | 0.349 |
| Geometric Mean | 25.1 | 32.7 | |
| 80% Confidence Interval | 18.2, 34.7 | 27.7, 38.7 | |
| CSerum 14 (mg/L) | |||
| n | 7 | 37 | 0.190 |
| Geometric Mean | 24.7 | 37.04 | |
| 80% Confidence Interval | 17.1, 35.5 | 31.6, 43.4 | |
| CSerum 70 (mg/L) | |||
| n | 4 | 21 | 0.533 |
| Geometric Mean | 24.5 | 32.9 | |
| 80% Confidence Interval | 13.9, 43.1 | 25.8, 42.1 | |
| AUCSerum (mg/L) | |||
| n | 11 | 38 | <0.001 |
| Geometric Mean | 15.4 | 33.8 | |
| 80% Confidence Interval | 12.4, 19.1 | 30.1, 37.9 | |
Based on ANOVA with outcome of the natural log-transformed pharmacokinetic measure.
Table 2.
Association between pharmacokinetic parameters and subject characteristics
| Baseline Characteristics Associated With Each PK Parametera | Parameter | p-value |
|---|---|---|
| CCSF 14 | ||
| Goodness of Fit (Coefficient of determination) | 0.62897 | |
| Viral load | −0.00081 | 0.0538 |
| Serum creatinine | −0.69993 | 0.0756 |
| CSerum 14 | ||
| None | None | None |
| CSerum 70 | ||
| Goodness of fit (coefficient of determination) | 0.41386 | |
| On HAART at baseline (No) | 3.71629 | 0.0017 |
| On HAART at baseline (No) * dose received through day 70 | −0.00177 | 0.0085b |
| On HAART at baseline (Yes)* dose received through day 70 | 0.00656 | |
| AUCSerum | ||
| Goodness of fit (coefficient of determination) | 0.55035 | |
| Baseline eGFR (mL/min/1.73 m2) | −0.01935 | 0.0011 |
| Baseline eGFR*dose received through day 70 (grams) | 0.00004 | 0.0042 |
|
| ||
| Post-Baseline Characteristics Associated With Each PK Parameterc | Parameter | p-value |
|
| ||
| CCSF 14 | ||
| Log (day 14 CSF WBC count) | −0.0013 | 0.0410 |
| Day 14 CrCl | −0.0110 | 0.1324 |
| CSerum 14 | ||
| Log (day 14 CSF WBC Count) | −0.3207 | 0.0238 |
| Day 14 CrCl | −0.0121 | 0.1495 |
| CSerum 70 | ||
| Day 70 eGFR | −0.0098 | 0.0049 |
| Day 70 CrCl | −0.0130 | 0.0222 |
| Day 70 Serum creatinine | 0.8965 | 0.0449 |
| AUCSerum | ||
| Log (day 14 CSF WBC Count) | −0.1819 | 0.0445 |
Based on linear regression models with the outcome of the natural log-transformed PK measure and adjusting for dose received.
P-value for test of interaction.
Based on linear regression models with the outcome of the natural log-transformed PK measure.
There was a strong relationship between dose received and CSerum14, CCSF14 and AUCSerum (p<0.001); but a weaker relationship between dose received and CSerum70 (p=0.126). Increased AUCSerum appeared associated with decreased mortality at day 70 as well as with the increased study composite endpoint success at Days 42 and 70 (Figure 1). Numerically, a similar trend was seen between the other pharmacokinetic measures and mortality at day 70 and the study composite endpoint at Days 42 and 70. However, the trend was not seen at Day 14.
Figure 1.
Odds ratios for clinical outcomes at each time point associated with each PK parameter. Lines represent 80 percent confidence intervals for the odds ratios. AUC=AUCSerum (mg/L), CSF14= ln(CCSF14 (mg/L)), Serum14= ln(CSerum14 (mg/L)), and Serum70= ln(CSerum70 (mg/L)). The number of successes and failures for the clinical outcome included in each model are provided at the bottom of the figure.
Discussion
To date, clinical trials attempting to predict adequate antifungal CNS pharmacokinetics for treatment of CNS fungal infections have been limited.3 BAMSG 3-01 demonstrated that fluconazole concentrations in the brain closely paralleled serum levels. The median percentage of CSF compared to serum fluconazole concentrations for the AmB+Fluc800 and AmB+Fluc400 arms were 93.7% and 94.6%, respectively after 14 days of antifungal treatment. These concentration ratios are consistent with previous results3, 7 but are higher than 70%, which is achieved in the absence of meningeal inflammation.8 Furthermore, CSerum14 and CCSF14 were found to be highly correlated. Therefore, we can surmise that the steady state of metabolism in both serum and CSF had been achieved. The increased serum concentration in patients receiving fluconazole 800 mg/day compared to those receiving standard dose of fluconazole over time may be explained by the elimination half-life of fluconazole following zero order kinetics and only 10% of elimination because of the metabolism.9
Fluconazole renal clearance has been found to be positively correlated with eGFR9. In the model for AUCSerum, decreased baseline eGFR was associated with high AUCSerum; however, the impact of baseline eGFR decreased as dose received increased, suggesting that non-renal elimination pathways may become increasingly important as the fluconazole dose increases. The other subject characteristics, including age and BMI, were not associated with pharmacokinetic parameters (data not shown). Major factors affecting fluconazole pharmacokinetic identified previously included renal insufficiency, aging, and drug-drug interactions from concurrent medication use. 2,9 Of note, increased pharmacokinetic concentration was associated with decreased day 14 CSF WBC count in BAMSG 3-01. Normally, the CSF WBC count increases due to inflammation of the CNS and disruption of the blood brain barrier. The CSF profiles of HIV and non-HIV infected patients are similar for conventional bacterial meningitis, but not cryptococcal meningitis. HIV-infected patients with cryptococcal meningitis are more likely to have a low CSF WBC count and are more likely to have a positive CSF culture.1
During the course of therapy, risk factors for death or poor clinical outcome for cryptococcal meningitis that have been identified previously included abnormal mental status, high CSF cryptococcal antigen titer, low CSF WBC count, disseminated cryptococcal infection, CSF fungal burden in the CSF and lack of flucytosine treatment. 10,11 While this study was not designed to formally assess the association between pharmacokinetic concentration and cryptococccal meningitis outcome, the findings revealed a tendency of association between high levels of fluconazole and favorable outcomes at days 42 and 70. These findings further support the potential benefits of high-dose fluconazole in the treatment of cryptococcal meningitis that were observed in the study’s primary analyses. To the best of our knowledge, this is the first clinical trial that has shown a relationship between pharmacokinetic measures and clinical outcome for antifungal treatment of a CNS fungal infection. However, the strongest relationship was between each outcome and SerumAUC. Because the calculation of SerumAUC includes fluconazole concentration at day 70; monitoring SerumAUC as a predictor for outcome at day 42 or 70 would not be reasonable. A larger study would be required to assess the benefits of prospectively monitoring early period fluconazole concentration as a predictor for outcome.
The target fluconazole concentration in serum and CSF for treatment of cryptococcal infection has not been defined so far. Based upon Clinical and Laboratory Standard Institute (CLSI) methodology for the treatment of candidal infections, an AUC:minimum inhibitory concentration (MIC) of at least 25 is required.12 Therefore, any future studies should focus on developing interpretive breakpoints requiring integration of the MIC distribution, pharmacokinetic and pharmacodynamic measures, and the relationship between in vitro activity and results from both in vivo and clinical trials.
BAMSG 3-01 provides promising pharmacokinetic data of fluconazole in terms of combined therapy of high-dose fluconazole (800 mg/day) with amphotericin B with regards to the relationship of CNS and serum fluconazole concentration with clinical outcomes. Although amphotericin B plus flucytosine is a preferred regimen in some countries, flucytosine is not available in many countries, especially in resource-constrained countries, that have HIV-related cryptococcal meningitis epidemics. Thus, our results apply and are beneficial to these particular countries and support a change in the early therapeutic approach to cryptococcal meningitis management in HIV-infected patients.
Acknowledgments
The authors wish to thank the additional members of the study group including Michele Morris, Jack Sobel, Mary Ellen Walker, Sanyaluk Parmanpol and Louise Zimmer as well as all patients in the study as well as the study coordinators and all other staff at the participating sites for their assistance in conducting the study.
Funding Statement
The study was supported in part with Federal Funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health under Contract Numbers N01 AI-15440 and N01 AI-15441 and 5R01AI1070091. Fluconazole study drug was generously donated by Pfizer, Inc. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States Government.
Footnotes
The abstract of this study was presented at the 16th Conference of Retroviruses and Opportunistic infections (CROI), Montreal 2009, P-175.
Transparency Declarations
W Manosuthi, P Chetchotisakd, T L Nolen, D Wallace, S Sungkanuparph, T Anekthananon, K Supparatpinyo, R A Larsen: No conflicts of interest.
P G Pappas has received research support from Pfizer, Merck, Schering Plough, and Astellas.
S G Filler has received research support from Pfizer and Merck, and owns equity in NovaDigm Therapeutics, Inc.
D Andes has received research support from Pfizer, Merck, and Astellas
W Manosuthi, P Chetchotisakd, S Sungkanuparph, T Anekthananon, K Supparatpinyo, R A Larsen, P G Pappas, and S G Filler participated in study design, collection of study data, and manuscript preparation.
T L Nolen and D Wallace participated in study design, analysis of study data, and manuscript preparation.
D Andes participated in designing the pharmacokinetic analyses and manuscript preparation.
References
- 1.Jarvis JN, Harrison TS. HIV-associated cryptococcal meningitis. Aids. 2007;21(16):2119–29. doi: 10.1097/QAD.0b013e3282a4a64d. [DOI] [PubMed] [Google Scholar]
- 2.Zonios DI, Bennett JE. Update on azole antifungals. Semin Respir Crit Care Med. 2008;29(2):198–210. doi: 10.1055/s-2008-1063858. [DOI] [PubMed] [Google Scholar]
- 3.Kethireddy S, Andes D. CNS pharmacokinetics of antifungal agents. Expert Opin Drug Metab Toxicol. 2007;3(4):573–81. doi: 10.1517/17425225.3.4.573. [DOI] [PubMed] [Google Scholar]
- 4.Andes D. Clinical utility of antifungal pharmacokinetics and pharmacodynamics. Curr Opin Infect Dis. 2004;17(6):533–40. doi: 10.1097/00001432-200412000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Pappas P, Nolen T, Chetchotisakd P, Larsen R, Manosuthi W, Filler S. A phase II trial of amphotericin B alone or combined with fluconazole in the treatment of HIV-associated cryptococcal meningitis. Clin Infect Dis. 2009;48(12) doi: 10.1086/599112. [DOI] [PubMed] [Google Scholar]
- 6.Harris SC, Wallace JE, Foulds G, Rinaldi MG. Assay of fluconazole by megabore capillary gas-liquid chromatography with nitrogen-selective detection. Antimicrob Agents Chemother. 1989;33(5):714–6. doi: 10.1128/aac.33.5.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thaler F, Bernard B, Tod M, et al. Fluconazole penetration in cerebral parenchyma in humans at steady state. Antimicrob Agents Chemother. 1995;39(5):1154–6. doi: 10.1128/aac.39.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debruyne D. Clinical pharmacokinetics of fluconazole in superficial and systemic mycoses. Clin Pharmacokinet. 1997;33(1):52–77. doi: 10.2165/00003088-199733010-00005. [DOI] [PubMed] [Google Scholar]
- 9.Debruyne D, Ryckelynck JP. Clinical pharmacokinetics of fluconazole. Clin Pharmacokinet. 1993;24(1):10–27. doi: 10.2165/00003088-199324010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Saag MS, Powderly WG, Cloud GA, et al. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. The NIAID Mycoses Study Group and the AIDS Clinical Trials Group. N Engl J Med. 1992;326(2):83–9. doi: 10.1056/NEJM199201093260202. [DOI] [PubMed] [Google Scholar]
- 11.Khawcharoenporn T, Apisarnthanarak A, Mundy LM. Treatment of cryptococcosis in the setting of HIV coinfection. Expert Rev Anti Infect Ther. 2007;5(6):1019–30. doi: 10.1586/14787210.5.6.1019. [DOI] [PubMed] [Google Scholar]
- 12.Pfaller MA, Diekema DJ, Sheehan DJ. Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing. Clin Microbiol Rev. 2006;19(2):435–47. doi: 10.1128/CMR.19.2.435-447.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]