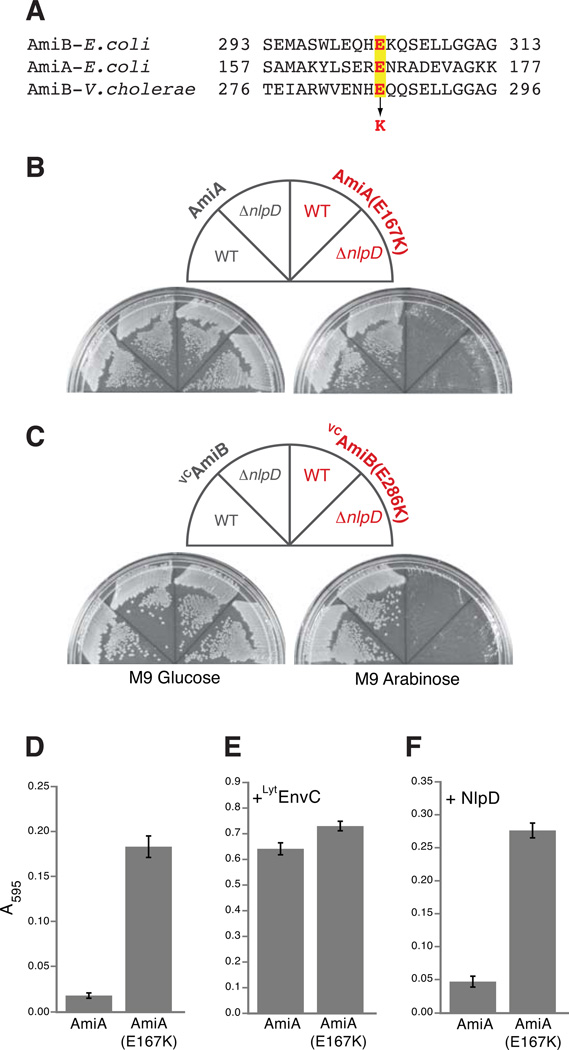
Figure 5. Lytic and PG hydrolase activities of other amidases with substitutions in their regulatory domains.
(A) Sequence alignment of a portion of the regulatory domains of E. coli AmiB, E. coli AmiA, and V. cholerae AmiB (VCAmiB). The highlighted glutamic acid (E) residues in red were changed to lysines (K) in AmiA and VCAmiB to yield AmiA(E167K) and VCAmiB(E286K), respectively. (B–C) As indicated, TB28[WT] or TB145[ΔnlpD] cells carrying AmiA, AmiA(E167K), VCAmiB, or VCAmiB(E286K) were spread on M9-CAA-Cm10 agar containing either 0.2% glucose or 0.2% arabinose. Plates were incubated overnight at 37°C and photographed. (D) Dye-release assay measuring basal PG hydrolase activity for AmiA(E167K) relative to AmiA (WT). Assays were performed as described in Fig. 4 except that reactions contained 1µM amidase with or without an additional 1µM of purified LytM factor as indicated.