Figure 4.
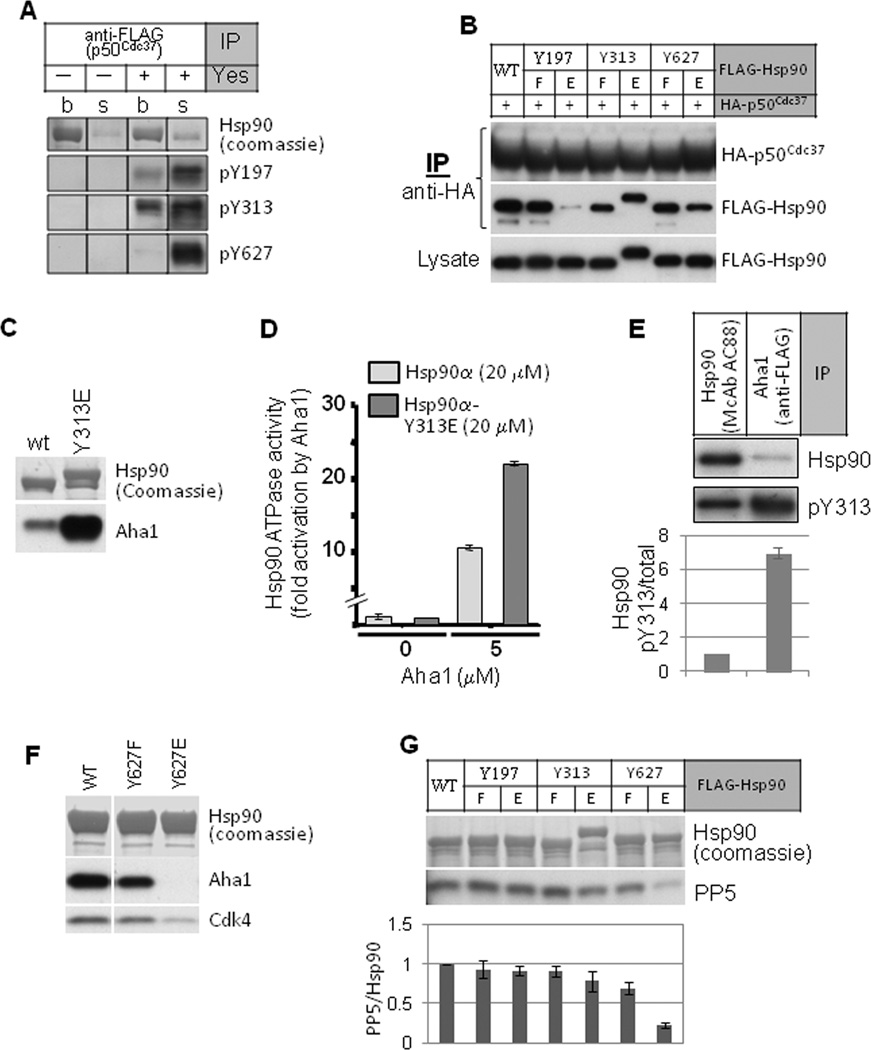
Hsp90 tyrosine phosphorylation regulates the association and dissociation of co-chaperones. A. FLAG-tagged p50Cdc37 was expressed in COS7 cells and immunoprecipitated. In vitro kinase assay with Yes kinase was as described. Endogenous Hsp90 phosphorylation on tyrosine residues was detected with site-specific antibodies (see Fig. S4 for antibody validation). “s” indicates Hsp90 released into the kinase assay buffer; “b” indicates Hsp90 remaining bound to p50Cdc37 immune complexes. B. Phosphomimetic mutation of Hsp90-Y197 disrupts p50Cdc37 association. HA-tagged p50Cdc37 was expressed in 293A cells together with indicated FLAG-tagged Hsp90 proteins, and p50Cdc37 was immunoprecipitated with anti-HA beads. Association of Hsp90 was detected with anti-FLAG antibody. C. Phosphomimetic mutation of Hsp90-Y313 promotes AHA1 association. FLAG-tagged Hsp90 was precipitated from transfected COS7 cells. Association of AHA1 was detected by western blot. D. AHA1 protein stimulates ATPase activity of Hsp90-Y313E to a greater extent than that of wild type Hsp90. The ATPase activity of purified Hsp90 proteins (20 µM) was determined in the presence or absence of AHA1 (5 µM). E. AHA1-associated Hsp90 is hyper-phosphorylated on Y313. FLAG-tagged AHA1 was expressed in 293A cells and immunoprecipitated with anti-FLAG antibody. Associated Hsp90 was examined for phosphorylation on Y313 using antibody recognizing pY313, and the signal was compared with Y313 phosphorylation of total Hsp90 immunoprecipitated from 293A cells. The pY313 signal intensity was normalized to the total Hsp90 signal intensity and graphically displayed. F. Phosphomimetic mutation of Hsp90-Y627 dissociates AHA1 and client kinase (Cdk4) from Hsp90. Indicated FLAG-tagged Hsp90 proteins were transiently expressed in 293A cells and immunoprecipitated with anti-FLAG. Association of endogenous AHA1 and Cdk4 was examined by western blot. G. Phosphomimetic mutation of Hsp90-Y627 disrupts PP5 association. Indicated FLAG-tagged Hsp90 proteins were expressed in 293A cells and immunoprecipitated with anti-FLAG beads. Association of the co-chaperone phosphatase PP5 was visualized with specific antibody. Loading equivalence was confirmed by Coomassie stain of immunoprecipitated Hsp90 proteins (see also Fig. S4). Please see Experimental Procedures for quantitation method.