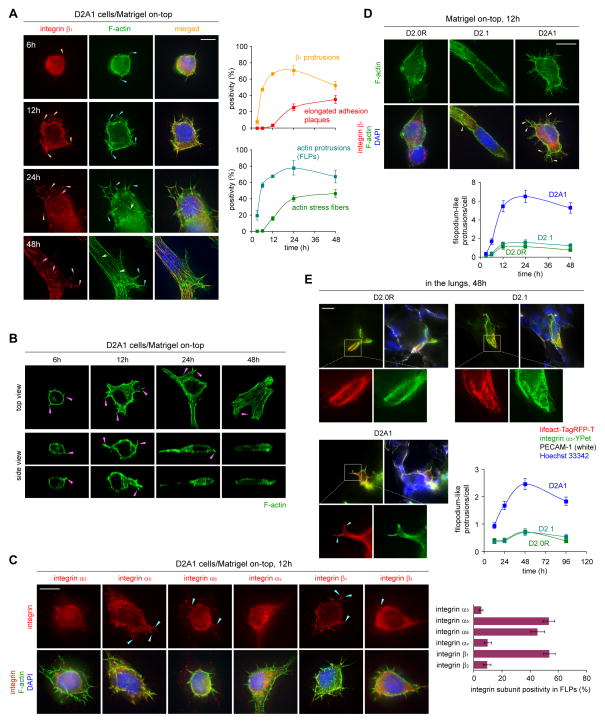
Figure 2. Filopodium-like protrusions that precede adhesion plaque assembly.
(A) Temporal development of elongated adhesion plaques. The localization of integrin β1 (red), F-actin (phalloidin; green) and nuclei (blue) was determined at indicated time points in the MoT-cultured D2A1 cells (left). Integrin β1-containing protrusions (orange arrowheads), elongated adhesion plaques (pink arrows), protrusions of F-actin (blue arrowheads), and actin stress fibers (green arrows) are indicated. The presence of these structures was quantified (right).
(B) Upward and downward projections of FLPs. Top and side views of the MoT-cultured D2A1 cells with F-actin-staining (green) are presented with indication of FLPs by the pink arrowheads.
(C) Localization of integrin subunits to FLPs. MoT-cultured D2A1 cells were stained for integrin subunits (red), F-actin (green) and nuclei (blue) (left). The accumulation of these subunits to FLPs is indicated (blue arrowheads). The probability with which the length of FLP shaft was covered by the integrin staining was plotted (right).
(D) FLP formation in MoT-cultured D2 cells. Integrin β1-containing FLPs are indicated by the yellow arrowheads (top). The number of FLPs per cell was plotted (bottom).
(E) In vivo FLP formation. D2 cells expressing lifeact-Tag-RFP-T (red) and integrin α5-YPet (green) were tail-vein injected into mice. Blood vessels (PECAM-1-staining; white) and nuclei (blue) were visualized together with these fluorescent proteins on lung sections, where FLPs are indicated (blue arrowheads). These cells are not surrounded by the PECAM-1-staining and therefore are likely to have extravasated into the lung parenchyma. The number of FLPs per cell was plotted (bottom-right).
Bars = 10 μm. Values = means ± SD (n = 3; A,C) or means ± SEM (n = 100; D,E).