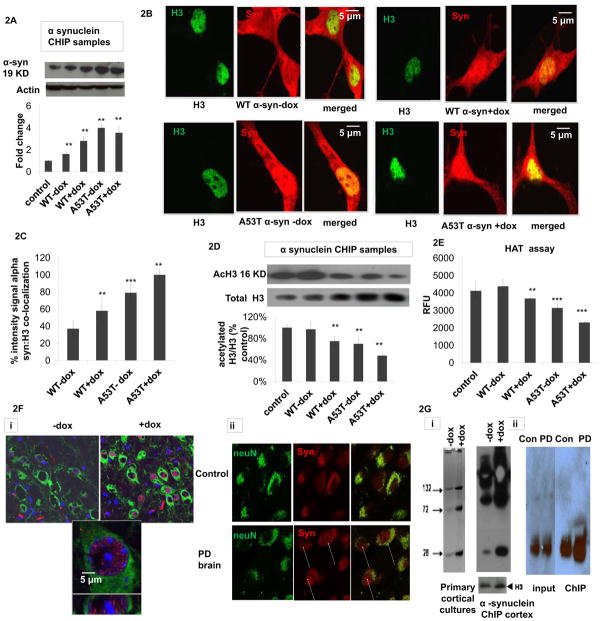
Fig 2. Oxidatively-induced increase in nuclear 3-synuclein coincides with increased chromatin binding in vitro and in vivo and in Parkinsonian neurons versus age-matched controls.
(A) Representative alpha-synuclein western blot of isolated alpha-synuclein ChIP samples from untransfected (control), wildtype (WT) and A53T alpha-synuclein (α-syn) transfected cells +/− dox (actin was used as loading control) and corresponding densitometric analysis (n=4). Values are expressed as mean ± SD; **p<0.01 versus control. (B) Representative fluorescent confocal images of dual alpha-synuclein (red, BD Transduction) versus histone (H3, Cell Signaling) immunocytochemistry (merged, yellow) of WT and A53T 3-synuclein PC12 +/− dox induction (−/+Dox). (C) Co-localization as quantitated by yellow versus green nuclei in 5 fields per chamber (n=2) using NIH imageJ free software. Values are expressed as mean ± SD; **p<0.01 versus WT-dox. (D) Representative acetylated H3 (acH3) and total H3 western blots of isolated nuclear α-synuclein ChIP samples from untransfected controls, wildtype (WT) and A53T alpha-synuclein (α-syn) transfected cells +/− dox and corresponding densitometric analysis of acH3 normalized to total H3 (n=3). Values are expressed as mean ± SD; **p<0.01 versus WT-dox group. (E) Biochemical histone actyl transferase (HAT) activity reported as relative fluorescence units (RFU) in nuclear fractions from untreated controls, WT or A53T α-synuclein cells +/−dox; values are expressed as mean ± SD; **p<0.01 & ***p<0.001 versus WT-dox. (F) Co-alpha-synuclein (red) and neuronal (NeuN, green) immunostaining in (i) mouse brain tissues −/+ induction of oxidative stress via doxycycline (-dox versus +dox) versus nuclear (blue) DAPI staining; lower panel is a higher magnification of the upper dox-treated panel and (ii) in brain tissues from PD patients versus age-matched controls (yellow, merged). Arrows denote presence of alpha-synuclein in the nuclei of PD neurons. Results shown are representative of those observed in 10 separate fields. (G) Alpha-synuclein ChIP was performed on (i) samples from either primary cortical cultures isolated from MAO-B transgenic mice (left panel) either treated with dox (D) or left untreated (-dox, 24 hrs) or on brain tissues (right panel) isolated from inducible transgenic MAO-B lines fed dox versus vehicle (+dox and -dox, 3 weeks) using the alpha-synuclein Ab Syn 205 (Cell Signaling) per the Abcam Protein A ChIP kit instructions. Immunoblots were probed with Ab Syn 205 and H3 antibody as a normalization control. Samples were run on a 10% Bis-Tris NuPAGE gel (Invitrogen), blotted and probed with either an alternate alpha-synuclein antibody (BD Biociences) or H3 antibody (Abcam). Bands represent synuclein multimers of higher MW; P>0.05,+dox versus -dox. (ii) ChIP was performed on chromatin IP’d from affected SN PD tissues versus age-matched controls; samples were subsequently analyzed by alpha-synuclein ChIP-western. IP = immunoprecipitation, I = 100% input; p>0.05, PD versus Con.