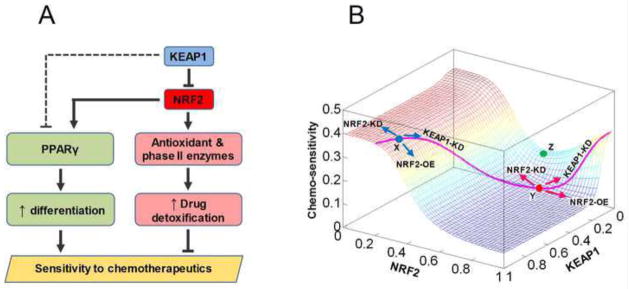
Fig. 9.
KEAP1 and NRF2 may modulate sensitivity of cells to chemotherapeutic agents through differentially regulating two opposing pathways. (A) In NSCLC cells, moderate downregulation of KEAP1 and activation of the canonical NRF2-ARE pathway due to mutations upregulate antioxidant and phase II enzymes without appreciably activating the PPARγ pathway. Augmented drug detoxification capacity reduces the sensitivity of cancer cells to chemotherapeutics. Further knockdown of KEAP1, which is already lower than in normal cells, would lift its inhibition on PPAR through an unknown mechanism (dashed line), permitting activation of PPARγ by NRF2. Increased PPARγ promotes cancer cell differentiation and inhibits proliferation, thus enhancing the killing effect of chemotherapeutic agents. (B) The mathematical model recapitulated the differential responses of normal cells and cancer cells to knockdown of KEAP1. The landscape describes how chemo-sensitivity changes as KEAP1 and NRF2 levels are independently varied. In normal cells (represented by location X, blue dot) containing high KEAP1 and low NRF2, knockdown of KEAP1 results in reduced sensitivity of cells to the toxicity of chemotherapeutic agents. In some cancer cells (represented by location Y, red dot) containing relatively lower KEAP1 and higher NRF2, further knockdown of KEAP1 would result in increased sensitivity to chemotherapeutics. In other cases, combined mutations in KEAP1 and NRF2 may push cells to a location such as Z (green dot), where both increasing and decreasing NRF2 would increase chemo-sensitivity. The pink curve delineates the changes in NRF2 and chemo-sensitivity as the KEAP1 level is independently varied in the model.