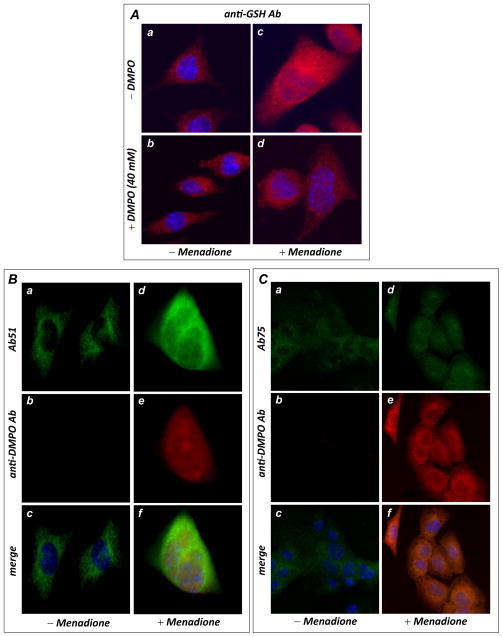
FIGURE 6.
In vivo protein radical formation and consequent S-glutathionylation from HL-1 myocytes under oxidative stress induced by menadione. A, immunomicroscopic analysis of S-glutathionylation in HL-1 using an anti-GSH monoclonal antibody. The upper panels (images a & c) are control HL-1 with or without menadione (40 μM) treatment, demonstrating enhancement of cellular S-glutathionylation by menadione. The lower panels (images b & d) are HL-1 with or without menadione treatment in the presence of DMPO (40 mM), demonstrating that DMPO outcompeted endogenous GSH for the protein radicals, decreasing cellular S-glutathionylation. B, Immunomicroscopic detection of Complex I protein radical formation using an anti-DMPO monoclonal antibody (red) and Ab51 (green). The left panels (images a, b & c) are control HL-1 with the addition of 40 mM DMPO trap, demonstrating that no detectable protein radical was formed. The right panels (images d, e, & f) are HL-1 treated with 40 μM of menadione with addition of the spin trap DMPO, demonstrating marked protein radical formation that colocalized with the Complex I 51 kDa subunit (green). C, same as B, except that cellular proteins were probed with Ab75 to show marked protein radical formation that colocalized with the 75 kDa subunit of Complex I.