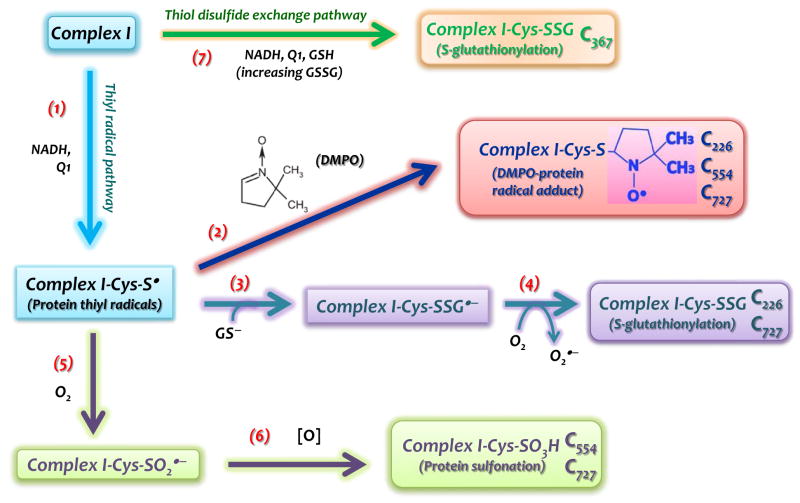
FIGURE 7.
Schematic delineation in which protein thiyl radical mediates Complex I S-glutathionylation and other oxidative modifications. The formation of Complex I thiyl radical intermediate (Complex I-Cys-S•) at the 75 kDa subunit is induced by oxidative attack of O2•− under the enzyme turnover conditions (Reaction 1). Immuno-spin trapping with DMPO (Reaction 2) and MS analysis reveal the residues of C226, C554, and C727 to be involved in the Complex I-Cys-S•. Intrinsic protein S-glutathionylation (Complex I-Cys-SSG) of complex I at C226 and C727 is facilitated by the reaction of Complex I-Cys-S• with glutathione thiolate ion (GS−) (Reaction 3) and subsequent production of O2•− (Reaction 4).. Complex I-Cys-S• can also mediate complex I S-sulfonation (Complex I-Cys-SO3H) at C554 and C727 via cysteine sulfinyl radical (Complex I-Cys-SO2•) and further reaction with oxidants (Reactions 5&6). Complex I-Cys-SSG can also occur independently of protein thiyl radical. GSSG, a product accumulated by the reaction system of complex I/NADH/Q1/GSH, may directly mediate Complex I-Cys-SSG at the C367 (Reaction 7) via thiol disulfide exchange.