Abstract
Gastric cancer remains one of the deadly diseases with poor prognosis. New classification of gastric cancers based on histologic features, genotypes and molecular phenotypes helps better understand the characteristics of each subtype, and improve early diagnosis, prevention and treatment. The objective of this article is to review the new classification of gastric cancers and the up-to-date guidance in the application of molecular testing.
Key Words: Gastric carcinoma, classification, histology, HER2, CDH1, DPD, molecular pathology
Introduction
Gastric cancer is the fourth most commonly diagnosed cancer and the second most common cause of cancer-related death worldwide (1,2). Although the incidence of gastric cancer has gradually decreased over the last half century, cancer at proximal stomach is on the rise (3,4). Today, gastric cancer is still the seventh most common cause of cancer-related death in the United States (5) and the prognosis of advanced gastric cancer remains poor. Gastric carcinogenesis is a multistep and multifactorial process. While the intestinal type of gastric cancer is often related to environmental factors such as Helicobacter pylori infection, diet, and life style, the diffuse type is more often associated with genetic abnormalities. Recent advances in molecular medicine have not only shed light on the carcinogenesis of gastric cancer, but also offered novel approaches regarding prevention, diagnosis and therapeutic intervention.
Classification of gastric carcinoma
Cancers at gastric cardia and gastroesophageal junction (GEJ)
Gastric carcinoma is clinically classified as early or advanced stage to help determine appropriate intervention, and histologically into subtypes based on major morphologic component. For the classification based on anatomic location, difficulty often arises when the tumor is located at proximal stomach or cardia, especially when the tumor also involves gastroesophageal junction (GEJ). It is not only because there are shared histologic features and immunophenotypes between the inflamed gastric cardiac mucosa due to Helicobacter infection and the metaplastic columnar epithelium-lined distal esophageal mucosa secondary to reflux disease, but also because there is no universal consensus regarding the anatomic definition of gastric cardia (6,7). Several classifications were proposed in order to address this issue. The scheme endorsed by the International Gastric Cancer Association separates gastric cancers into type I, type II and type III, to represent the tumors at distal esophagus, at cardia and at the stomach distal to cardia, respectively (8). This classification, however, has not clearly defined the criteria for each of these anatomic locations. Most recently, the 7th Edition of the TNM classification by American Joint Committee on Cancer (AJCC) has simplified the classification of the carcinoma at proximal stomach based on the location of tumor epicenter and the presence or absence of GEJ involvement (9). The tumor is to be stage grouped as esophageal carcinoma if its epicenter is in the lower thoracic esophagus or GEJ, or within the proximal 5 cm of stomach (i.e., cardia) with the tumor mass extending into GEJ or distal esophagus. If the epicenter is >5 cm distal to the GEJ, or within 5 cm of GEJ but does not extend into GEJ or esophagus, it is stage grouped as gastric carcinoma (9). This classification, although easy for pathologists to follow, could still face some challenges. For example, a bulky gastric cardiac cancer with its epicenter 4 cm below GEJ will still be diagnosed and classified as an esophageal tumor if the proximal end of tumor extends into GEJ by only 0.5 cm (even if the distal end of tumor is 4 cm from the epicenter extending into the stomach). For the operating surgeon who sees the tumor in situ, it may be difficult for him or her to accept this tumor as an esophageal cancer. In addition, a recent retrospective study by Huang et al. shows that cardiac carcinoma involving GEJ or distal esophagus is more appropriately classified and staged as gastric rather than esophageal cancers, at least in the Chinese population (10). In that study, cardiac carcinomas were staged according to the depth of invasion, status of positive lymph nodes and distant metastasis, as both gastric and esophageal tumors. When the tumor stage is studied and compared with cumulative survival, the findings support that it is more appropriately to group and stage cardiac cancers as stomach in origin (10). To better separate gastric cardiac carcinoma from esophageal or GEJ malignancy, more studies are apparently needed, such as a larger patient sample, molecular profiling of the tumor, clinical follow up data, and defining the tumor location after neoadjuvant therapy as to determine whether the initially bulky tumor was more “gastric” or more “GEJ/esophagus” in origin.
Early and advanced gastric carcinoma
Early gastric carcinoma is defined as invasive carcinoma confined to mucosa and/or submucosa, with or without lymph node metastases, irrespective of the tumor size (11). Most early gastric carcinomas are small, measuring 2 to 5 cm in size, and often located at lesser curvature around angularis. Some early gastric carcinoma can be multifocal, often indicative of a worse prognosis. Grossly, early gastric carcinoma is divided into Type I for the tumor with protruding growth, Type II with superficial growth, Type III with excavating growth, and Type IV for infiltrating growth with lateral spreading. Type II tumor is further divided to IIa (elevated), IIb (flat) and IIc (depressed), as proposed by the Japanese Endoscopic Society (12). A more recent Paris classification has endorsed three gross patterns for superficial neoplastic lesions in gastrointestinal tract. Grossly and endoscopically, the tumor is classified as Type 0-I for polypoid growth (which is subcategorized to 0-Ip for pedunculated growth and 0-Is for sessile growth), Type 0-II for nonpolypoid growth (which is subcategorized into Type 0-IIa for slightly elevated growth, Type 0-IIb for flat growth, and Type 0-IIc for slightly depressed growth), and Type 0-III for excavated growth (13). Histologically, the most common forms of early gastric carcinoma are well differentiated, mostly with tubular and papillary architecture. The distinction between well-differentiated carcinoma and high grade dysplasia or carcinoma in situ can be challenging when only mucosal tissue is available for histologic assessment. Intramucosal invasion may not be as easily confirmed as an invasive carcinoma into submucosa where stromal desmoplasia is usually evident. The distinction between intramucosal carcinoma and carcinoma in situ or high grade dysplasia is important, as the intramucosal carcinoma of stomach, unlike the intramucosal carcinoma in the colon, does metastasize. Generally, the useful histologic features of intramucosal invasion are single tumor cells in the lamina propria and significantly fused neoplastic glands of various sizes. The prognosis of early gastric carcinoma is excellent, with a 5 years survival rate as high as 90% (14). In contrast, the advanced gastric carcinoma which invades into muscularispropria or beyond carries a much worse prognosis, with a 5 years survival rate at about 60% or less (15). The gross appearance of advanced gastric carcinomas can be exophytic, ulcerated, infiltrative or combined. Based on Borrmann’s classification, the gross appearance of advanced gastric carcinomas can be divided into type I for polypoid growth, type II for fungating growth, type III for ulcerating growth, and type IV for diffusely infiltrating growth which is also referred to as linitisplastica in signet ring cell carcinoma when most of gastric wall is involved by infiltrating tumor cells. Histologically, advanced gastric carcinoma often demonstrates marked architectural and cytological heterogeneity, with several co-existing histologic growth patterns. The distinction between early and advanced gastric carcinoma before resection is clinically important because it helps decide if a neoadjuvant (pre-operative) therapy which has shown to improve disease free survival and overall survival (16,17) is warranted. While the macroscopic appearance is informative, the most accurate pre-operative staging information is generally obtained with endoscopic ultrasonography (EUS) and computer tomography (CT) (18).
Histologic classification of gastric carcinomas
Histologically, gastric carcinoma demonstrates marked heterogeneity at both architectural and cytologic level, often with co-existence of several histologic elements. Over the past half century the histologic classification of gastric carcinoma has been largely based on Lauren’s criteria, in which intestinal type and diffuse type adenocarcinoma are the two major histologic subtypes, plus indeterminate type as uncommon variant (18). The relative frequencies are approximately 54% for intestinal type, 32% for the diffuse type, and 15% for the indeterminate type (19). There are indications that the diffuse type gastric carcinoma is more often seen in female and young individuals (20,21), while the intestinal type adenocarcinoma is more often associated with intestinal metaplasia and Helicobacter pylori infection (22,23).
The 2010 WHO classification recognizes four major histologic patterns of gastric cancers: tubular, papillary, mucinous and poorly cohesive (including signet ring cell carcinoma), plus uncommon histologic variants (24). The classification is based on the predominant histologic pattern of the carcinoma which often co-exists with less dominant elements of other histologic patterns.
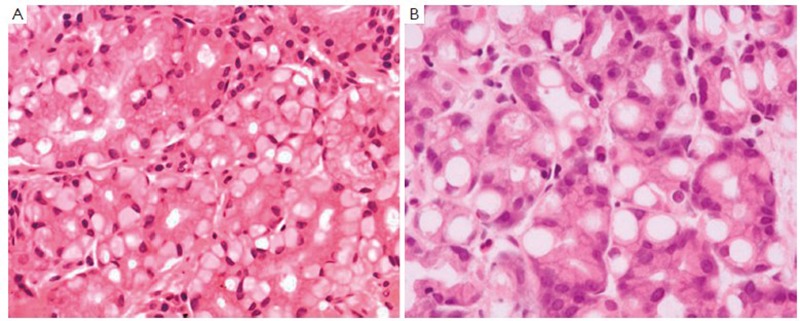
Tubular adenocarcinoma is the most common histologic type of early gastric carcinoma (Figure 1). It tends to form polypoid or fungating masses grossly, and histologically demonstrates irregularly distended, fused or branching tubules of various sizes, often with intraluminal mucus, nuclear and inflammatory debris.
Figure 1.
Tubular adenocarcinoma. Irregular-shaped and fused neoplastic glands with intraluminal mucus and debris
Papillary adenocarcinoma is another common histologic variant often seen in early gastric carcinoma. It tends to affect older people, occur in the proximal stomach, and is frequently associated with liver metastasis and a higher rate of lymph node involvement. Histologically, it is characterized by epithelial projections scaffolded by a central fibrovascular core.
Mucinous adenocarcinoma accounts for 10% of gastric carcinoma. Histologically it is characterized by extracellular mucinous pools which constitute at least 50% of tumor volume (Figure 2). The tumor cells can form glandular architecture and irregular cell clusters, with occasional scattered signet ring cells floating in the mucinous pools.
Figure 2.
Mucinous adenocarcinoma. Clusters and scattered tumor cells floating in the abundant extracellular mucin pools
Signet ring cell carcinoma (Figure 3) and other poorly cohesive carcinomas are often composed of a mixture of signet ring cells and non-signet ring cells. Poorly cohesive non-signet ring tumor cells are those that morphologically resemble histiocytes, lymphocytes, and plasma cells. Those tumor cells can form irregular microtrebaculae or lace-like abortive glands, often accompanied by marked desmoplasia in the gastric wall and with a grossly depressed or ulcerated surface. When it occurs at the antropyloric region with serosal involvement, the carcinoma tends to have lymphovascular invasion and lymph node metastasis. Because signet ring cell and other poorly cohesive carcinomas at antroplyoric region have a propensity to invade duodenum via submucosal and subserosal routes including subserosal and submucosal lymphatic spaces, special attention needs to be paid to those routes when a distal margin frozen section is requested at the time of surgical resection. Special stains such as cytokeratin immunohistochemistry can help detect morphologically occult signet ring cells in the lamina propria. One important differential diagnosis of neoplastic signet ring cells in gastric mucosa is benign pseudo-signet ring cells which can remarkably mimic signet ring cell carcinoma (Figure 4). Those pseudo-signet ring cells sometimes can demonstrate cytological atypia, even with mitoses. However, those pseudo-signet ring cells do not reveal invasive pattern with reticulin stain which highlights pseudo-signet ring cells confined within basement membrane with intact acinar architecture (Figure 5) (25).
Figure 3.
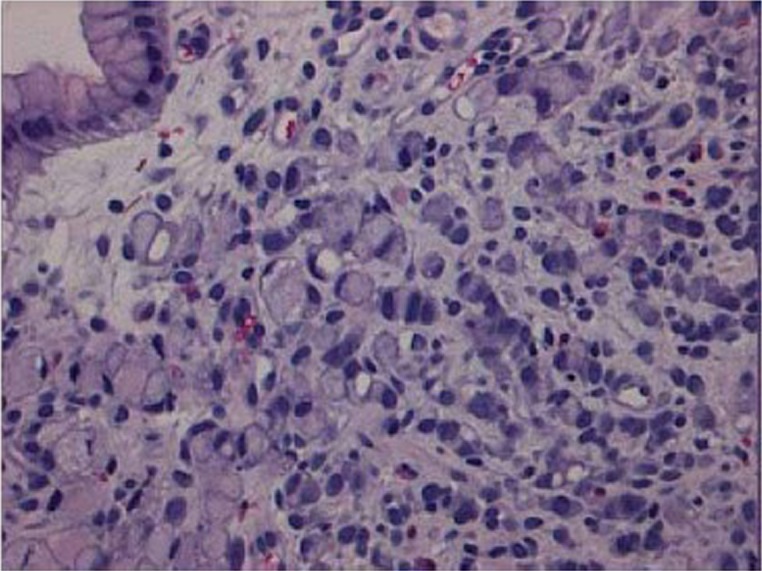
Signet ring cell carcinoma. Signet ring carcinoma cells are predominantly at the superficial lamina propria
Figure 4.
Pseudo-signet ring cells. The cytoplasm of pseudo-signet ring cells are vacuolated (A) and pale (B) (photos are courtesy of Dr. Caroline Hughes)
Figure 5.
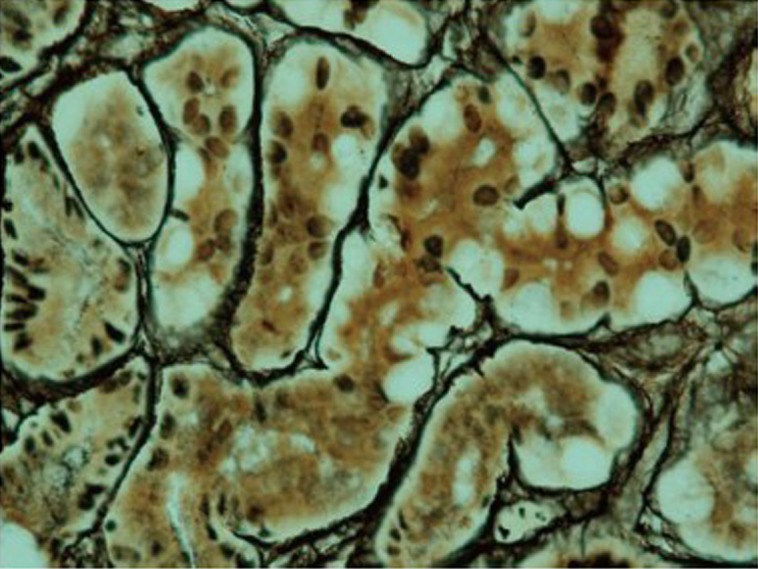
Pseudo-signet ring cells are confined within basement membrane and maintain intact acinar structure with reticulin stain (photo is courtesy of Dr. Caroline Hughes)
In addition to the above four major histologic subtypes, WHO classification also endorses other uncommon histologic variants, such as adenosquamous carcinoma, squamous carcinoma, hepatoid adenocarcinoma, carcinoma with lymphoid stroma, choriocarcinoma, parietal cell carcinoma, malignant rhabdoid tumor, mucoepidermoid carcinoma, paneth cell carcinoma, undifferentiated carcinoma, mixed adeno-neuroendocrine carcinoma, endodermal sinus tumor, embryonal carcinoma, pure gastric yolk sac tumor and oncocytic adenocarcinoma, all listed in Table 1, with Lauren’s classification for comparison.
Table 1. Gastric adenocarcinoma classification systems.
| WHO (2010) | Lauren (1965) |
|---|---|
| Papillary adenocarcinoma Tubular adenocarcinoma Mucinous adenocarcinoma |
Intestinal type |
| Signet-ring cell carcinoma And other poorly cohesive carcinoma |
Diffuse type |
| Mixed carcinoma |
Indeterminate type |
| Adenosquamous carcinoma Squamous cell carcinoma Hepatoid adenocarcinoma Carcinoma with lymphoid stroma Choriocarcinoma Carcinosarcoma Parietal cell carcinoma Malignant rhabdoid tumor Mucoepidermoid carcinoma Paneth cell carcinoma Undifferentiated carcinoma Mixed adeno-neuroendocrine carcinoma Endodermal sinus tumor Embryonal carcinoma Pure gastric yolk sac tumor Oncocytic adenocarcinoma |
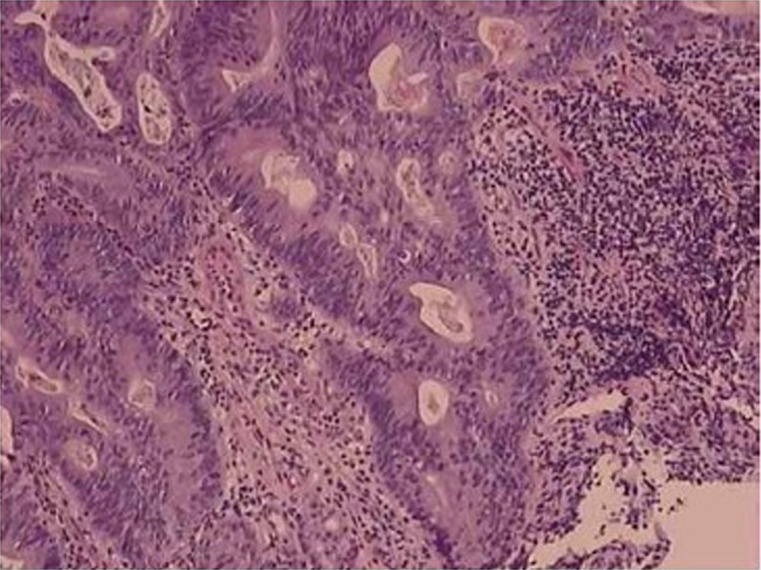
Gastric carcinoma with lymphoid stroma (medullary carcinoma) is one of the uncommon subtypes. It occurs more commonly in proximal stomach and generally follows a less aggressive clinical course. Histologically, this type of carcinoma is characterized by a sharply demarcated advancing margins composed of irregular nests or sheets of polygonal tumor cells associated with a prominent lymphoid infiltrate in a non-desmoplasticstroma. It is interesting that over 80% of gastric carcinomas with lymphoid stroma are Epstein-Barr virus (EBV) positive (26,27), and EBV is only identified in the malignant and dysplastic cells but not in the normal epithelial cells (28). The finding has raised the hope for tumor cell targeting, especially after studies show that Bortezomib, a proteasome inhibitor, can induce EBV kinase by activating EBV lytic protein expression in the infected tumor cells, which in turn renders the infected cells more susceptible to killing by other agents (29). Another group of gastric carcinomas with lymphoid stroma are those that demonstrate high microsatellite instability (30,31), resulting from defective function of DNA mismatch repair proteins, usually hMLH1 or hMSH2, but rarely hMSH6 (30,32-34). The number of tumor-infiltrating lymphocytes, while significantly higher than the one in non-microsatellite instability-high cancers, is lower than that in EBV positive carcinoma (34). This group of carcinoma is usually intestinal type by Lauren’s classification, and often affects the elderly, with a lower pTNM stage and a low risk of lymph node metastasis. It was suggested that microsatellite instability-high status and EBV infection were the variables which rendered the carcinoma a better prognosis. However, the claims have not been substantiated by other studies. More recent study reveals that the high number of tumor-infiltrating lymphocytes is the only favorable prognostic factor independent of EBV infection and microsatellite instability-high status (34). Also in this investigation, neither EBV positivity nor microsatellite instability-high alone was proved to be an independently favorable prognostic factor. Interestingly, EBV positivity and microsatellite instability-high status, while both share the feature of prominent tumor-infiltrating lymphocytes, are rarely concomitant, suggesting the two are unrelated and involved in distinct underlying pathways in carcinogenesis.
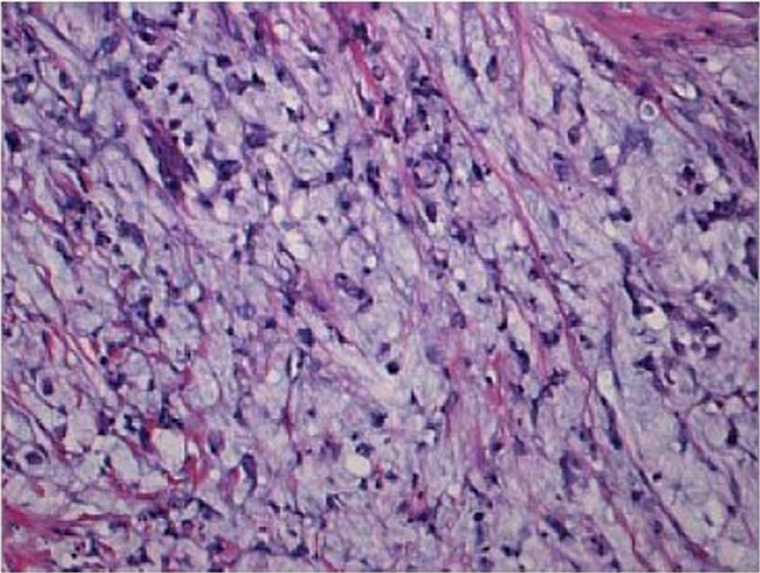
Micropapillary carcinoma of stomach is a newly recognized histologic variant characterized by small papillary clusters of tumor cells without a distinct fibrovascular core (Figure 6). The micropapillary features are often noted in the deep advancing edge of tumor, surrounded by an empty space mimicking retraction artifact. Micropapillary carcinoma of stomach, as its counterpart at other organs, tends to form endolymphatic tumor emboli and metastasize to lymph nodes. However, the overall survival of gastric micropapillary carcinoma, unlike that in other organs, seems to be not significantly different from conventional gastric adenocarcinoma, although the result may be due to the small patient sample in that study (11 patients) (35). Because of the high incidence of lymphatic invasion and nodal metastasis (up to 82%) (35,36), it is advised that conservative treatment such as endoscopic resection not be used for gastric carcinoma with invasive micropapillary components.
Figure 6.
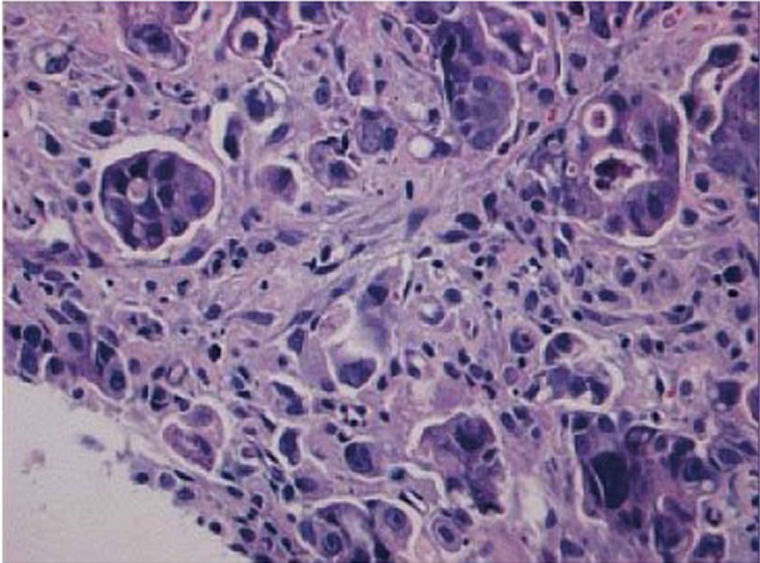
Micropapilary adenocarcinoma. Small papillary clusters of tumor cells devoid of fibrovascular core and surrounded by empty spaces
Application of molecular pathology in gastric carcinoma
An accumulation of genetic and molecular abnormalities occurs during gastric carcinogenesis, including activation of oncogenes, overexpression of growth factors/receptors, inactivation of tumor suppression genes, DNA repair genes and cell adhesion molecules (37), loss of heterogeneity and point mutations of tumor suppressor genes, and silencing of tumor suppressors by CpG island methylation (38). The revelation and understanding of the molecular events and pathways have led to the application of molecular pathology in the prevention, early diagnosis, tumor classification and therapeutic intervention. The applications of molecular testing such as the testing of CDH1 gene for hereditary diffuse gastric carcinoma (HDGC) and of HER2 expression in gastric cancers have had significant impact on medical practice, and become standard patient care.
Hereditary diffuse gastric carcinoma (HDGC)
About 10% of gastric carcinomas show familial clustering but only approximately 1-3% of gastric carcinomas arise from inherited gastric cancer predisposition syndromes (39), such as hereditary diffuse gastric carcinoma (HDGC), familial adenomatous polyposis, hereditary nonpolyposis colorectal carcinoma (or Lynch syndrome), juvenile polyposis syndrome, Peutz-Jeghers syndrome, Li-Fraumeni syndrome and gastric hyperplastic polyposis (40-42). HDGC is an autosomal dominant disorder with high penetrance. Approximately 30% of individuals with HDGC have a germline mutation in the tumor suppressor gene E-cadherin or CDH1 (43). The inactivation of the second allele of E-cadherin through mutation, methylation, and loss of heterozygosity eventually triggers the development of gastric cancer (44,45). To diagnose HDGS, two or more cases of diffuse gastric carcinoma in first or second degree relatives must be documented, with at least one diagnosed before the age of 50; or there are three or more documented cases of diffuse gastric carcinoma in first or second degree relatives, regardless of the age of onset (46,47).
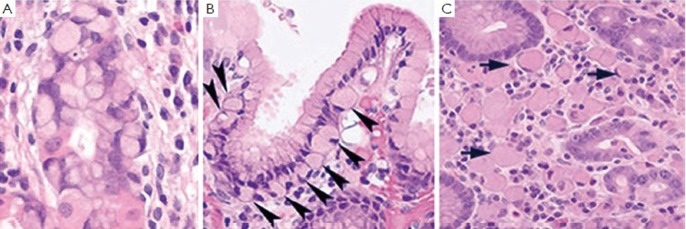
The histologic phenotype of HDGC in early stage includes patchy intramucosal signet ring carcinoma cells in the lamina propria and its unique feature of carcinoma in situ associated with pagetoid spread of tumor cells along the preserved basement membrane (Figure 7). The lesion can be multifocal but usually starts at the junction of antrum and body. The tumor cells often demonstrate hyperchromatic nuclei, with occasional mitoses. Because it is difficult to diagnose HDGC at an early stage both histologically and endoscopically, and because the penetrance of CDH1 mutation is high, with the carrier of this gene conferring over 80% life time risk of gastric carcinoma (47), prophylactic total gastrectomy after confirmation through CDH1 molecular testing is the only recommended way to save patients’ lives. According to the updated recommendations for CDH1 testing by International Gastric Cancer Consortium, family members of the following are the candidates for CDH1 testing (48): (I) Two family members with gastric carcinoma, one of which is confirmed diffuse gastric cancer; (II) Three family members with gastric carcinoma in first or second degree relatives including one with diffuse gastric cancer; (III) One member with diffuse gastric cancer before the age of 40; (IV) Personal or family history of diffuse gastric cancer and lobular breast cancer including one diagnosed before 50.
Figure 7.
A.In situ signet ring carcinoma cells confined within basement membrane; B. Pagetoid spread of signet ring cells (arrow heads) below the preserved surface epithelium; C. Focus of intramucosal signet ring cell carcinoma (arrows) in the lamina propria (all three photos are courtesy of Dr. Rebecca Fitzgerald)
If in situ signet ring cell carcinoma with pagetoid spread is identified adjacent to diffuse type gastric cancer and confirmed by expert GI pathologists, the patient should also be tested for CDH1 mutation, because the histologic features have not been reported in sporadic form of gastric carcinoma (49). The confirmation of HDGC through CDH1 mutation can help family members decide if they should consider the similar testing.
Because approximately 4% of these mutation positive families exhibit large germline deletions of CDH1 that cannot be detected by conventional DNA analysis (50), large genomic rearrangements should be sought in addition to conventional direct sequencing. It is also recommended that CDH1 genetic testing on blood for germline mutations should be performed in Clinical Laboratory Improvement Laboratory (CLIA)-certified molecular diagnostic laboratories or research laboratories with expertise in CDH1 gene analysis (48).
In addition to prophylactic total gastrectomy, annual mammography and breast MRI from the age of 35 years are recommended for women with HDGC, due to their increased risk of lobular breast cancer (51).
Human epidermal growth factor receptor 2
Human epithelial growth factor receptor 2 (HER2), a member of the human epidermal growth factor receptor (EGFR) family, is a proto-oncogene located on chromosome region 17q21. It encodes a 185 kDtransmembrane tyrosine kinase receptor protein that regulates signal transduction in cell proliferation, differentiation and survival (52,53). HER2 gene amplification was described in gastric carcinoma after its discovery in breast cancer (54). With immunohistochemical stain, it was found that the rate of HER2 overexpression in gastric adenocarcinoma is 12% in a Japanese series (55) and 22.1% in more recent studies (56-58). HER2 overexpression is more often noted in intestinal type carcinoma (57,59) and in the carcinomas located at proximal stomach or cardia and gastroesophageal junction (24-35%) than in the remaining stomach (9.5% to 21%) (19,59,60). In addition, HER2 status in the carcinomas of stomach and GEJ is relatively homogeneous and rarely shows significant modification from primary site to metastatic foci (61).
Recently, a large scale phase III international clinical trial called ToGA showed that the humanized monoclonal antibody against HER2, Trastuzumab (Herceptin), when combined with chemotherapy (capocitabine or 5-fluorouracil and cisplatin), could effectively prolong overall survival and progression-free survival, and increases the response rate in HER2 positive advanced gastric carcinoma (57). On the basis of these findings, the regulatory approval for trastuzumab was granted in October 2010 in the United States for patients with HER2 positive metastatic adenocarcinoma of stomach or gastroesophagical junction. Now, it is recommended that all patients with gastric cancers should routinely be tested for the HER2 status at the initial diagnosis (57,62).
While HER2 positive status in gastric carcinoma is also defined as either IHC3+ or IHC2+ plus positive FISH, similar to breast cancers, there are several differences in the evaluation of HER2 status in gastric cancers. In gastric or GEJ cancers, only 5 clustered positive cancer cells in a biopsy tissue or a minimum 10% of positive neoplastic cells in a surgical resection specimen are required for defining 3+ score, on the condition that the immunohistochemical stain reveals intense complete, basolateral, or lateral membranous reactivity (62). In order to archive accurate and reproducible HER2 scoring, it is essential that the interpretation of HER2 expression is strictly based on the criteria originally reported in the Trastuzumab for gastric cancer study, which was published and listed in Table 2 (57).
Table 2. WHO 2010 classification and grading of PETs (5,21).
| Classification/Grade | Mitotic count (per 10 hpf) | Ki 67 Index (%) |
|---|---|---|
| NET-G1 |
<2 |
<3 |
| NET-G2 |
2-20 |
3-20 |
| NEC-G3 | >20 | >20 |
NET: neuroendocrine tumor; NEC: neuroendocrine carcinoma. hpf: high power field; 10 HPF =2 mm2, at least 40 fields (at 400× magnification) evaluated in areas of highest mitotic density
In addition, a panel of expert pathologists from the European Union and the rest of the world recommend that if immunohistochemistry is used as the initial test, any specimen type (either surgical resection or biopsy) with <10% strongly stained tumor cells should be subjected to confirmatory in situ hybridization testing to preclude false-negative results (62). If the sample is poorly preserved, shows nonspecific staining at cytoplasm and nuclei of the tumor cells, or reveals staining at benign mucosa with intestinal metaplasia, the sample should be retested by FISH to exclude false positive results (62).
Based on the results from ToGA study, the levels of HER2 protein predicts well for the response of gastric carcinoma to Trastuzumab. On the other hand, the tumors with positive HER2 amplification but with low or negative HER2 expression do not respond well to Trastuzumab. Therfore, immunohistochemistry is recommended to be used as the initial testing methodology, and FISH or silver in situ hybridization used to retest immunohistochemistry 2+ cases (62).
Dihydropyrimidine dehydrogenase
Dihydropyrimidine dehydrogenase (DPD) is the rate-limiting enzyme in uracil catabolism, and is also the main enzyme involved in the degradation of structurally related compounds like 5-Fluorouracil (5-FU), a widely used drug in treating different kinds of tumor including gastric carcinoma. True deficiency of DPD affects approximately 5% of the overall population (63). Patients with DPD deficiency are at significantly increased risk of developing severe and potentially fatal neutropenia, mucositis and diarrhea (63-65) when treated with 5-FU or capecitabine. In addition, 3% to 5% of the population has a partial DPD deficiency due to sequence variations in DPYD gene, which potentially limits their ability to fully metabolize the drug, thereby resulting in toxicity (66-68). Many studies have addressed and identified the mutations of DPYD and epigenetic alterations of DPYD as the causes of lower levels of DPD or DPD deficiency. Subsequently, different tests have been developed in order to identify the people at risk of DPD deficiency, in the hope that the test results could eventually provide clinical guidance. One of the tests to identify the people with DPD deficiency is DPYD genotyping to detect the important mutations such as DPYD 2A (or IVS14+1 G>A) (66,69). While the individuals with positive DPYD mutation have an increased risk for DPD deficiency, DPD deficiency is also noted in the people with wild type PDYD, because epigenetic alteration, such as methylation at the regulatory region of PDYP promoter can cause lower DPD level without the mutation at DNA level (70). To make issue more complicated is that the uracil catabolic pathway involves several other enzymes such as dihydropyrimidinase (DHP) (71) and beta-urreidopropionase (BUP1) (72,73). The mutations of those genes which are at the downstream of DPD also impair uracil catabolism. Therefore, uracil breath test which involves DPD, DHP, and DUP1 may reveal more clinical information of potential toxicity in the patients who receive 5-FU treatment (74), because it evaluates the integrity of the entire catabolic pathway of uracil which cannot be archived by PDYD genotyping alone.
Despite the fact that PDYD genotyping is informative for identifying patients with an increased risk of toxicity to 5-FU treatment, and despite the large numbers of studies which attempt to identify molecular predictors of response and toxicity to treatment, none of the tests and molecular markers thus far have been proven to be reliable in prospective clinical trials, and unlike CDH1 and HER2 testing, none of those tests have been validated to permit their use as standard of care in 5-FU therapy. Many questions still remain unanswered and many components in the entire metabolic pathways of FU remain unaddressed. For example, DPD deficiency was noted only in a small percentage of patients with severe 5-FU toxicity, leaving a large numbers of patients with an unexplainable molecular basis of toxicity (75). In predicting who will develop toxicity when treated with 5-FU or capecitabine, much more work has to be done (76).
In conclusion, while gastric cancer remains a deadly disease, the discoveries of new molecular markers, genetic and epigenetic alteration, and novel pharmacogenetic traits have helped improve patients care, fostered hope and led new directions of cure. The newest WHO classification of gastric carcinoma is by far the most comprehensive, describing the morphologic characteristics of each subtype in detail. Hopefully, it will help understand the clinicopathologic entity of each subtype by correlating its histologic feature with molecular profiling and clinical behavior. It is encouraging that the discoveries of some pharmacogenetic traits have opened the door for individualized medicine, promising the future medicine to be more effective and less toxic because it is based on the molecular fingerprint not only of each tumor but of each human being. Nevertheless, many challenges remain. Some claims to attempt pharmacogenetic prediction based on the pattern of single nuclear polymorphsim (SNP) may be premature and have not been fully validated. Caution should be exercised as some of claims may be biased and could lead to harmful consequences (77,78).
Acknowledgments
We thank Dr. Rebecca Fitzgerald (Hutchinson/MRC Research Center, Cambridge, UK) for kindly providing us the photos in Figure 7, and Dr. Caroline Hughes (Academic Center, Oxford, UK) for kindly providing us the photos in Figures 4 and 5. We also thank Ms. Cheryl Devine for her effort and help in retrieving the cases of gastric carcinoma for photomicrograph.
Disclosure: The authors declare no confict of interest.
References
- 1.Parkin DM. International variation. Oncogene 2004;23:6329-40 [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917 [DOI] [PubMed] [Google Scholar]
- 3.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998;83:2049-53 [PubMed] [Google Scholar]
- 4.Blot WJ, Devesa SS, Kneller RW, et al. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 1991;265:1287-9 [PubMed] [Google Scholar]
- 5.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90 [DOI] [PubMed] [Google Scholar]
- 6.Chandrasoma PT, Der R, Ma Y, et al. Histology of the gastroesophageal junction: an autopsy study. Am J Surg Pathol 2000;24:402-9 [DOI] [PubMed] [Google Scholar]
- 7.Genta RM, Huberman RM, Graham DY. The gastric cardia in Helicobacter pylori infection. Hum Pathol 1994;25:915-9 [DOI] [PubMed] [Google Scholar]
- 8.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 1998;85:1457-9 [DOI] [PubMed] [Google Scholar]
- 9.Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manuel. 7 th ed. New York: Springer, 2010. [Google Scholar]
- 10.Huang Q, Shi J, Feng A, et al. Gastric cardiac carcinomas involving the esophagus are more adequately staged as gastric cancers by the 7th edition of the American Joint Commission on Cancer Staging System. Mod Pathol 2011;24:138-46. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton R, Aatonen LA. Tumors of Digestive System.Lyon:IARC; 2000:39-52. [Google Scholar]
- 12.Murakami T.Patholomorphological diagnosis. Definition and gross classification of early gastric cancer. Gann Monohr Cancer Res 1971;11:53-5 [Google Scholar]
- 13.The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. GastrointestEndosc 2003;58:S3-43 [DOI] [PubMed] [Google Scholar]
- 14.Everett SM, Axon AT. Early gastric cancer in Europe. Gut 1997;41:142-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshikawa K, Maruyama K.Characteristics of gastric cancer invading to the proper muscle layer--with special reference to mortality and cause of death. Jpn J ClinOncol 1985;15:499-503 [PubMed] [Google Scholar]
- 16.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectablegastroesophageal cancer. N Engl J Med 2006;355:11-20 [DOI] [PubMed] [Google Scholar]
- 17.Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectablegastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J ClinOncol 2011;29:1715-21 [DOI] [PubMed] [Google Scholar]
- 18.Hwang SW, Lee DH, Lee SH, et al. Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J Gastroenterol Hepatol 2010;25:512-8 [DOI] [PubMed] [Google Scholar]
- 19.Polkowski W, van Sandick JW, Offerhaus GJ, et al. Prognostic value of Laurén classification and c-erbB-2 oncogene overexpression in adenocarcinoma of the esophagus and gastroesophageal junction. Ann Surg Oncol 1999;6:290-7 [DOI] [PubMed] [Google Scholar]
- 20.Lauren P.The two histological main types of gastric carcinoma: diffuse and so called intestinal-type carcinoma: an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965;64:31-49 [DOI] [PubMed] [Google Scholar]
- 21.Caldas C, Carneiro F, Lynch HT, et al. Familial gastric cancer: overview and guidelines for management. J Med Genet 1999;36:873-80 [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneko S, Yoshimura T.Time trend analysis of gastric cancer incidence in Japan by histological types, 1975-1989. Br J Cancer 2001;84:400-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsonnet J, Vandersteen D, Goates J, et al. Helicobacter pylori infection in intestinal- and diffuse-type gastric adenocarcinomas. J Natl Cancer Inst 1991;83:640-3 [DOI] [PubMed] [Google Scholar]
- 24.Lauwers GY, Carneiro F, Graham DY. Gastric carcinoma. In: Bowman FT, Carneiro F, Hruban RH, eds. Classification of Tumours of the Digestive System. Lyon:IARC;2010. In press. [Google Scholar]
- 25.Hughes C, Greywoode G, Chetty R.Gastric pseudo-signet ring cells: a potential diagnostic pitfall. Virchows Arch 2011;459:347-9 [DOI] [PubMed] [Google Scholar]
- 26.Wu MS, Shun CT, Wu CC, et al. Epstein-Barr virus-associated gastric carcinomas: relation to H. pylori infection and genetic alterations. Gastroenterology 2000;118:1031-8 [DOI] [PubMed] [Google Scholar]
- 27.Wang HH, Wu MS, Shun CT, et al. Lymphoepithelioma-like carcinoma of the stomach: a subset of gastric carcinoma with distinct clinicopathological features and high prevalence of Epstein-Barr virus infection. Hepatogastroenterology 1999;46:1214-9 [PubMed] [Google Scholar]
- 28.Truong CD, Feng W, Li W, et al. Characteristics of Epstein-Barr virus-associated gastric cancer: a study of 235 cases at a comprehensive cancer center in U.S.A. J Exp Clin Cancer Res 2009;28:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu DX, Tanhehco Y, Chen J, et al. Bortezomib-induced enzyme-targeted radiation therapy in herpesvirus-associated tumors. Nat Med 2008;14:1118-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halling KC, Harper J, Moskaluk CA, et al. Origin of microsatellite instability in gastric cancer. Am J Pathol 1999;155:205-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliveira C, Seruca R, Seixas M, et al. The clinicopathological features of gastric carcinomas with microsatellite instability may be mediated by mutations of different “target genes”: a study of the TGFbeta RII, IGFII R, and BAX genes. Am J Pathol 1998;153:1211-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu CW, Chen GD, Jiang KC, et al. A genome-wide study of microsatellite instability in advanced gastric carcinoma. Cancer 2001;92:92-101 [DOI] [PubMed] [Google Scholar]
- 33.Thibodeau SN, French AJ, Roche PC, et al. Altered expression of hMSH2 and hMLH1 in tumors with microsatellite instability and genetic alterations in mismatch repair genes. Cancer Res 1996;56:4836-40 [PubMed] [Google Scholar]
- 34.Grogg KL, Lohse CM, Pankratz VS, et al. Lymphocyte-rich gastric cancer: associations with Epstein-Barr virus, microsatellite instability, histology, and survival. Mod Pathol 2003;16:641-51 [DOI] [PubMed] [Google Scholar]
- 35.Roh JH, Srivastava A, Lauwers GY, et al. Micropapillary carcinoma of stomach: a clinicopathologic and immunohistochemical study of 11 cases. Am J Surg Pathol 2010;34:1139-46 [DOI] [PubMed] [Google Scholar]
- 36.Ushiku T, Matsusaka K, Iwasaki Y, et al. Gastric carcinoma with invasive micropapillary pattern and its association with lymph node metastasis. Histopathology 2011;59:1081-9 [DOI] [PubMed] [Google Scholar]
- 37.Yasui W, Sentani K, Motoshita J, et al. Molecular pathobiology of gastric cancer. Scand J Surg 2006;95:225-31 [DOI] [PubMed] [Google Scholar]
- 38.Kitaura K, Chone Y, Satake N, et al. Role of copper accumulation in spontaneous renal carcinogenesis in Long-Evans Cinnamon rats. Jpn J Cancer Res 1999;90:385-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliveira C, Suriano G, Ferreira P, et al. Genetic screening for familial gastric cancer. Hered Cancer Clin Pract 2004;2:51-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasen HF, Wijnen JT, Menko FH, et al. Cancer risk in families with hereditary nonpolyposis colorectal cancer diagnosed by mutation analysis. Gastroenterology 1996;110:1020-7 [DOI] [PubMed] [Google Scholar]
- 41.Keller G, Rudelius M, Vogelsang H, et al. Microsatellite instability and loss of heterozygosity in gastric carcinoma in comparison to family history. Am J Pathol 1998;152:1281-9 [PMC free article] [PubMed] [Google Scholar]
- 42.Varley JM, McGown G, Thorncroft M, et al. An extended Li-Fraumeni kindred with gastric carcinoma and a codon 175 mutation in TP53. J Med Genet 1995;32:942-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pharoah PD, Guilford P, Caldas C, International Gastric Cancer Linkage Consortium Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 2001;121:1348-53 [DOI] [PubMed] [Google Scholar]
- 44.Barber M, Murrell A, Ito Y, et al. Mechanisms and sequelae of E-cadherin silencing in hereditary diffuse gastric cancer. J Pathol 2008;216:295-306 [DOI] [PubMed] [Google Scholar]
- 45.Oliveira C, Sousa S, Pinheiro H, et al. Quantification of epigenetic and genetic 2nd hits in CDH1 during hereditary diffuse gastric cancer syndrome progression. Gastroenterology 2009;136:2137-48 [DOI] [PubMed] [Google Scholar]
- 46.Caldas C, Carneiro F, Lynch HT, et al. Familial gastric cancer: overview and guidelines for management. J Med Genet 1999;36:873-80 [PMC free article] [PubMed] [Google Scholar]
- 47.Oliveira C, Bordin MC, Grehan N, et al. Screening E-cadherin in gastric cancer families reveals germline mutations only in hereditary diffuse gastric cancer kindred. Hum Mutat 2002;19:510-7 [DOI] [PubMed] [Google Scholar]
- 48.Fitzgerald RC, Hardwick R, Huntsman D, et al. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 2010;47:436-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oliveira C, Moreira H, Seruca R, et al. Role of pathology in the identification of hereditary diffuse gastric cancer: report of a Portuguese family. Virchows Arch 2005;446:181-4 [DOI] [PubMed] [Google Scholar]
- 50.Oliveira C, Senz J, Kaurah P, et al. Germline CDH1 deletions in hereditary diffuse gastric cancer families. Hum Mol Genet 2009;18:1545-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masciari S, Larsson N, Senz J, et al. Germline E-cadherin mutations in familial lobular breast cancer. J Med Genet 2007;44:726-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akiyama T, Sudo C, Ogawara H, et al. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science 1986;232:1644-6 [DOI] [PubMed] [Google Scholar]
- 53.Popescu NC, King CR, Kraus MH. Localization of the human erbB-2 gene on normal and rearranged chromosomes 17 to bands q12-21.32. Genomics 1989;4:362-6 [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto T, Ikawa S, Akiyama T, et al. Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature 1986;319:230-4 [DOI] [PubMed] [Google Scholar]
- 55.Yonemura Y, Ninomiya I, Yamaguchi A, et al. Evaluation of immunoreactivity for erbB-2 protein as a marker of poor short term prognosis in gastric cancer. Cancer Res 1991;51:1034-8 [PubMed] [Google Scholar]
- 56.Yokota J, Yamamoto T, Toyoshima K, et al. Amplification of c-erbB-2 oncogene in human adenocarcinomas in vivo. Lancet 1986;1:765-7 [DOI] [PubMed] [Google Scholar]
- 57.Van Cutsem E, Kang Y, Chung H, et al. Efficacy results from the ToGA trial: A phase III study of trastuzumab added to standard chemotherapy (CT) in first-line human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer (GC). J ClinOncol 2009;27:LBA 4509.
- 58.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97 [DOI] [PubMed] [Google Scholar]
- 59.Tanner M, Hollmén M, Junttila TT, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol 2005;16:273-8 [DOI] [PubMed] [Google Scholar]
- 60.Gravalos C, Jimeno A.HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol 2008;19:1523-9 [DOI] [PubMed] [Google Scholar]
- 61.Marx AH, Tharun L, Muth J, et al. HER-2 amplification is highly homogenous in gastric cancer. Hum Pathol 2009;40:769-77 [DOI] [PubMed] [Google Scholar]
- 62.Rüschoff J, Hanna W, Bilous M, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol 2012;25:637-50 [DOI] [PubMed] [Google Scholar]
- 63.Lee A, Ezzeldin H, Fourie J, et al. Dihydropyrimidine dehydrogenase deficiency: impact of pharmacogenetics on 5-fluorouracil therapy. Clin Adv Hematol Oncol 2004;2:527-32 [PubMed] [Google Scholar]
- 64.Schwab M, Zanger UM, Marx C, et al. Role of genetic and nongenetic factors for fluorouracil treatment-related severe toxicity: a prospective clinical trial by the German 5-FU Toxicity Study Group. J Clin Oncol 2008;26:2131-8 [DOI] [PubMed] [Google Scholar]
- 65.Deenen MJ, Tol J, Burylo AM, et al. Relationship between single nucleotide polymorphisms and haplotypes in DPYD and toxicity and efficacy of capecitabine in advanced colorectal cancer. Clin Cancer Res 2011;17:3455-68 [DOI] [PubMed] [Google Scholar]
- 66.Gross E, Busse B, Riemenschneider M, et al. Strong association of a common dihydropyrimidine dehydrogenase gene polymorphism with fluoropyrimidine-related toxicity in cancer patients. PLoS One 2008;3:e4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amstutz U, Froehlich TK, Largiadèr CR. Dihydropyrimidine dehydrogenase gene as a major predictor of severe 5-fluorouracil toxicity. Pharmacogenomics 2011;12:1321-36 [DOI] [PubMed] [Google Scholar]
- 68.Kim SR, Park CH, Park S, et al. Genetic polymorphisms associated with 5-Fluorouracil-induced neurotoxicity. Chemotherapy 2010;56:313-7 [DOI] [PubMed] [Google Scholar]
- 69.Johnson MR, Diasio RB. Importance of dihydropyrimidine dehydrogenase (DPD) deficiency in patients exhibiting toxicity following treatment with 5-fluorouracil. Adv Enzyme Regul 2001;41:151-7 [DOI] [PubMed] [Google Scholar]
- 70.Zhang X, Soong R, Wang K, et al. Suppression of DPYD expression in RKO cells via DNA methylation in the regulatory region of the DPYD promoter: a potentially important epigenetic mechanism regulating DPYD expression. Biochem Cell Biol 2007;85:337-46 [DOI] [PubMed] [Google Scholar]
- 71.Thomas HR, Ezzeldin HH, Guarcello V, et al. Genetic regulation of dihydropyrimidinase and its possible implication in altered uracil catabolism. Pharmacogenet Genomics 2007;17:973-87 [DOI] [PubMed] [Google Scholar]
- 72.Van Kuilenburg AB, Van Lenthe H, Assmann B, et al. Detection of beta-ureidopropionase deficiency with HPLC-electrospray tandem mass spectrometry and confirmation of the defect at the enzyme level. J Inherit Metab Dis 2001;24:725-32 [DOI] [PubMed] [Google Scholar]
- 73.Thomas HR, Ezzeldin HH, Guarcello V, et al. Genetic regulation of beta-ureidopropionase and its possible implication in altered uracil catabolism. Pharmacogenet Genomics 2008;18:25-35 [DOI] [PubMed] [Google Scholar]
- 74.Mattison LK, Ezzeldin H, Carpenter M, et al. Rapid identification of dihydropyrimidine dehydrogenase deficiency by using a novel 2-13C-uracil breath test. Clin Cancer Res 2004;10:2652-8 [DOI] [PubMed] [Google Scholar]
- 75.Diasio RB, Johnson MR. Dihydropyrimidine dehydrogenase: its role in 5-fluorouracil clinical toxicity and tumor resistance. Clin Cancer Res 1999;5:2672-3 [PubMed] [Google Scholar]
- 76.Ezzeldin HH, Diasio RB. Predicting fluorouracil toxicity: can we finally do it? J Clin Oncol 2008;26:2080-2 [DOI] [PubMed] [Google Scholar]
- 77.Baggerly KA, Combes KR. Deriving chemosensitivity from cell lines: forensic bioinformatics and reproducible research in high-throughput biology. Ann Appl Stat 2009;3:1309-34 [Google Scholar]
- 78.Valachis A, Mauri D, Neophytou C, et al. Translational medicine and reliability of single-nucleotide polymorphism studies: can we believe in SNP reports or not? Int J Med Sci 2011;8:492-500 [DOI] [PMC free article] [PubMed] [Google Scholar]