Abstract
Background
Blood doping represents one main trend in doping strategies. Blood doping refers to the practice of boosting the number of red blood cells (RBCs) in the bloodstream in order to enhance athletic performance, by means of blood transfusions, administration of erythropoiesis-stimulating substances, blood substitutes, natural or artificial altitude facilities, and innovative gene therapies. While detection of recombinant EPO and homologous transfusion is already feasible through electrophoretic, mass spectrometry or flow cytometry-based approaches, no method is currently available to tackle doping strategies relying on autologous transfusions.
Materials and methods.
We exploited an in vitro model of autologous transfusion through a 1:10 dilution of concentrated RBCs after 30 days of storage upon appropriate dilution in freshly withdrawn RBCs from the same donor. Western blot towards membrane Prdx2 and Percoll density gradients were exploited to assess their suitability as biomarkers of transfusion.
Results
Membrane Prdx2 was visible in day 30 samples albeit not in day 0, while it was still visible in the 1:10 dilution of day 30 in day 0 RBCs. Cell gradients also highlighted changes in the profile of the RBC subpopulations upon dilution of stored RBCs in the fresh ones.
Discussion.
From this preliminary in vitro investigation it emerges that Prdx2 and RBC populations might be further tested as candidate biomarkers of blood doping through autologous transfusion, though it is yet to be assessed whether the kinetics in vivo of Prdx2 exposure in the membrane of transfused RBCs will endow a sufficient time-window to allow reliable anti-doping testing.
Keywords: blood doping, red blood cell, population, peroxiredoxin 2
Introduction
One recent trend in illicit doping practices regards the adoption of blood doping, which is forbidden by the World Anti-Doping Agency (WADA)1. Blood doping refers to the practice of boosting the number of red blood cells (RBCs) in the bloodstream in order to enhance athletic performance, by means, for example, of transfusions. A wide group of illicit practices goes under the name of blood doping, including blood transfusions, administration of erythropoiesis-stimulating substances, blood substitutes, natural or artificial altitude facilities, and innovative gene therapies2. Blood transfusion for doping purposes is an extremely straightforward, practical and effective means of increasing an athlete’s red blood-cell supply in advance of competition, which became rather popular in the 1970s. Nonetheless it has suddenly declined upon the introduction and widespread diffusion of recombinant human erythropoietin (rEPO) among elite endurance athletes in late 80’s.3 As RBCs carry oxygen from the lungs to the muscles, an increase in the overall number of circulating RBCs might result in the improvement of an athlete’s aerobic capacity (VO2 max) and endurance 4. Blood doping has become rather widespread especially in those sports where other doping strategies based on hormone stimulation (erythropoietins)5 or other drugs are no longer feasible, due to the rise of new anti-doping approaches. Most recently, following implementation of reliable tests to screen for erythropoiesis-stimulating substances in 20013, trends in blood doping have come back to origins, with blood transfusions making a strong resurgence. Doping by blood transfusion can be classified as homologous, where the blood is infused into someone other than the donor, and autologous, where the blood donor and transfusion recipient are the same. The former case produces more clinically relevant side effects, while it is easily detectable using current antidoping protocols based on erythrocyte phenotyping by flow cytometry6.
Since the donor and recipient blood are identical in autologous blood doping, this is less risky, though much more challenging to detect. Indirect strategies, relying on significant deviations from individual hematological profiles following autologous blood donation and reinfusion, are currently being investigated.
Other than in the modalities of collection (homologous versus autologous), RBCs could also be differentially stored at 4 °C (hypothermique storage) or glycerolized and thus frozen in order to be cryostored. RBCs uniquely suited to this process because they can be concentrated, frozen and later thawed with little loss of viability (haemolysis below 1% and 24h in vivo survival above the 75% thresholds), though both procedures hold different biological drawbacks7–9. In an autologous transfusion, the athlete’s own RBCs are harvested well in advance of competition and then re-introduced before a critical event. For some time after the harvesting the athlete may be anemic. However, cryostorage is rather expensive and thus only top athletes are thought to be able to afford such a technology, while hypothermique storage is thought to be more diffused.
From a logistical standpoint, either type of transfusion requires the athlete to surreptitiously transport cold or frozen RBCs, thaw (for the latter) and re-infuse them in a non-clinical setting and then dispose of the medical paraphernalia.
However, also other blood doping approaches are not free of health hazards. Excessive use of the rEPO hormone, for example, can raise hematocrit above 70% which can cause polycythemia, increase blood viscosity and raise the likelihood of heart suffering from excessive stress, which could result in fatal outcomes. Indeed, rEPO use is a suspect in nearly 20 deaths in 4 years in European cyclists. In the 1998 Tour de France, a team was ejected for using rEPO and six other teams quit the race, while in recent years, diverse endurance and sprint athletes have been caught or accused of using rEPO10.
While rEPO could be now easily detected through electrophoretic and MS approaches11, indirect testing might allow also monitoring of indirect effects of rEPO in order to reveal doping in athletes long time after assumption of the drug. Such a detection strategy focuses on the monitoring of RBC-related parameters, including hematocrit (HCT) and the concentration of hemoglobin (Hb).
On the other hand, at the moment there is no official methodology available to detect autologous blood transfusions. Total haemoglobin mass measurements12 and the detection of metabolites of blood bags plasticizers (di(2-ethylhexyl) phthalate -DEHP) in urine13 have been recently proposed as valid strategies to tackle blood doping episodes.
However, no definitive approach is currently available and the search for alternative strategies is still an open issue.
Literature has provided a great deal of data about hypothermically stored blood9,14–16. Recent publications from our group have documented irreversible modifications taking place at the protein level as storage progresses at 4 °C degrees, such as the accumulation of membrane protein fragments or aggregates8, the accumulation at the membrane level of oxidative stress-related proteins such as peroxiredoxin (Prdx) 217 and the alteration of its oligomeric state18. The goal of the present paper is to provide preliminary results about testing of the hypothesis whether the anomalous and irreversible changes in protein patterns (in particular Prdx2), which we could outline in our previous investigations on blood storage9,17,18, might also represent a suitable marker for in vitro aging of RBCs could be hopefully adopted also as markers of doping transfusion practices in athletes.
Materials and methods
Sample collection
Whole blood (56.25 mL ± 10%) was collected from healthy volunteer donors into CPD anticoagulant (7.875 mL). After separation of plasma and buffy coat by centrifugation, RBCs were suspended in 12.5 mL of SAG-M (Saline, Adenine, Glucose, Mannitol) additive solution. We studied RBC units collected from 4 donors (male=2, female=2, age 35±8.5 [mean±S.D.]) in Rome (Italy), upon signing of informed consent according to the declaration of Helsinki. It is worthwhile to stress that the experiment was a scale-down of a routine donation/transfusion workflow, since only 1/8 of the volume of a routine donation (56.25 mL vs 450 mL) was collected from the same donor twice, once at day 0 (which would be stored for 30 days), and the second time at 30 days after the first withdrawal, in order to obtain a fresh day 0 control to be exploited in 1:10 dilutions, as specified below, without any complication to the donor.
RBC units were stored under standard blood bank conditions (4±2 °C) and samples were removed aseptically for the analysis at day 0 or upon 30 days of storage.
RBC dilution: in vitro model for blood transfusion
An in vitro model for dilution 1:10 of 30 days old RBCs in day 0 RBCs from the same donor has been designed as to simulate dilutions of RBCs in the bloodstream of the recipient/transfusing athlete. Indeed the final HCT of RBCs from processed whole blood and stored in CPD-SAGM is approximately 60 percent. A unit of packed RBCs contains approximately 150–200 mL of RBCs. Since the average whole blood volume in an adult male is approximately 5 liters, with hematocrit around 45%, the approximate volume of circulating RBCs is around 2.250 liters. Thus, a reliable in vitro model would be designed as to perform a 1:10 dilution as follows: after 30 days of storage RBCs will be concentrated (through removal of the additive solution via centrifugation) and diluted in 10 volumes of day 0 RBCs enriched from whole blood.
RBC protein extraction
Extraction of membrane and cytosol proteins from human erythrocyte has been performed following the conventional method as described by Olivieri et al.19 with minor modifications. Erythrocytes were isolated by centrifuging twice at 1,000 × g for 10 min. Packed cells were washed three times in 5 mM phosphate buffer pH 8.0, containing 0.9% w/v NaCl; then, they were centrifuged at 300 x g for 10 min, at 4 °C. Erythrocytes were resuspended in 1 mL PBS containing 100 mM N-ethylmaleimide (NEM), to avoid possible oxidation artifacts during cell preparation20. After 15 min of incubation at room temperature, cells were pelleted and then lysed with 9 vol of cold 5 mM phosphate buffer pH 8.0 containing 1 mM EDTA, 1 mM phenylmethanesulfonyl fluoride (PMSF) and 100 mM NEM. Cytosol was collected after centrifugation at 17,000 × g for 20 min at 4 °C and its protein content was estimated by the DC protein assay method (Bio-Rad, Hercules, CA, USA). Membranes were washed with the same buffer until free of hemoglobin: in order to remove non-specifically membrane-bound cytosolic proteins, membranes were further washed for three times with 0.9% w/v NaCl and collected at 17,000×g, for 20 min at 4 °C. Protein content was estimated by the bicinchoninic acid method21 and the membrane samples were exploited for the subsequent analyses.
1D-SDS-PAGE gel electrophoresis
Electrophoretic analyses of the RBC membrane proteins were carried out on a continuous system of polyacrilamide gels in the presence of sodium dodecyl sulphate (SDS-PAGE) using a non-reducing 14% acrylamide gel (30 μg protein/lane) according to Laemmli21. To prepare RBC membranes for electrophoresis, membrane suspensions were treated with an equal volume of solubilization buffer (0.125M Tris HCl pH 6.8, 4% SDS, 20% glycerol, 0.053% bromophenol blue) containing either 200 mM DTT when working under reducing conditions, or 100 mM NEM in oxidizing conditions. Proteins were thus blotted for western blot analysis towards Prdx2, as specified below.
Western blot analysis against Prdx2 in 1:10 diluted long-stored (30 days) RBCs in day 0 RBC concentrates
Proteins separated through 1D-SDS-PAGE were electrophoretically transferred to a polyvinylidene difluoride membrane. To reduce the likelihood of false positives, blocking has been performed for 2 hours at room temperature in 5% (wt/vol) non-fat dried milk in Tris-buffered saline. Incubation with antibodies anti-human Prdx2 was performed overnight at 4 °C in 1% (wt/vol) bovine serum albumin in Tris-buffered saline/0.1%Tween 20. Bands were detected with goat anti-rabbit horseradish peroxidase using enhanced chemiluminescence reagents and digitized with a high-resolution scanner (ImageScanner II, GE Healthcare). Quantification of band intensities was performed with an ad hoc analytic software (Quantity One 4.6.3, Bio-Rad), using an internal control of human recombinant Prdx2 protein. The amount of Prdx2 of each sample was determined as a ratio between the sample value and the internal control.
Separation of RBC populations
Density-fractionated RBCs were prepared as previously reported (Cryo e Blood Transfusion nostro), using Percoll (Sigma-Aldrich, St. Louis, MO, USA) discontinuous gradients as described by Bosch et al.22. Briefly, the gradient was built up in five layers of 2 mL containing 80% (1.096 g/mL), 71% (1.087 g/mL), 67% (1.083 g/mL), 64% (1.080 g/mL) Percoll, respectively, buffered with buffer A (26.3 g/L bovine serum albumin, 132 mmol/L NaCl, 4.6 mmol/L KCl, and 10 mmol/L HEPES pH 7.1). RBCs were washed with buffer B (9 mmol/L Na2HPO4, 1.3 mmol/L NaH2PO4, 140 mmol/L NaCl, 5.5 mmol/L glucose, and 0.8 g/L bovine serum albumin) and diluted with 1 vol of buffer A. One-half milliliter of this suspension was layered on the Percoll gradient and separation was achieved after 15 minutes of centrifugation at 3,000 rpm at room temperature. Fractions were collected by careful pipetting and extensively rinsed with buffer B to remove any residual Percoll.
Results and discussions
Blood doping through rEPO could be now assessed rather easily through electrophoretic approaches11 or monitoring of HCT and Hb. Indeed, normal HCT values are within the range of 41–50% in adult men and 36–44% in adult women, while normal Hb levels are 14–17 g/dL of blood in men and 12–15 g/dL in women. Although for most healthy persons the two measurements are in close agreement, in athletes assuming rEPO as blood doping two cases might occur: both the values anomalously increase as a result of doping, or the athletes are biologically prone to have higher-than-normal values for both the parameters. The Union Cycliste Internationale (UCI), for example, imposes a 15-day suspension from racing on any male athlete found to have an HCT above 50% and hemoglobin concentration above 17 grams per deciliter (g/dL). A few athletes naturally have high RBC concentrations (polycythemia), which they must demonstrate through a series of consistently high HCT and Hb results over an extended period of time. All these parameters are routinely included in the biological passport of each cycling professional, and their fluctuations monitored in order to identify eventual assumption of illicit substances.
A recent, more sophisticated method of analysis against doping through rEPO, which has not yet reached the level of an official standard, is to compare the numbers of mature and immature RBCs in an athlete’s circulation. If a high number of mature RBCs is not accompanied by a high number of immature RBCs (reticulocytes) it suggests that the mature RBCs were artificially introduced by transfusion11. rEPO use can also lead to a similar RBC profile because a preponderance of mature RBCs tends to suppress the formation of reticulocytes. A measure known as the “stimulation index” or “off-score” has been proposed based on an equation involving hemoglobin and reticulocyte concentrations. A normal score is 85–95 and scores over 133 are considered evidence of doping. The stimulation index is defined as Hb (g/L) minus sixty times the square root of the percentage of RBCs identified as reticulocytes.
Doping through homologous blood transfusion is easily detectable using current antidoping protocols based on erythrocyte phenotyping by flow cytometry6. On the other hand, detection of blood doping through autologous blood transfusion is still challenging and no definitive method currently exists, while two approaches have been recently proposed including total Hb mass measurement and urine DEHP levels12,13. Therefore, novel biomarkers which could be suitable to detect autologous transfusions might be individuated through the translation of the ongoing in-depth investigations on RBC storage.
The introduction of plastic bags, the diffusion of new collection and additive solutions other than the introduction of leukocyte filtering strategies have dramatically improved safety and efficacy of RBC concentrates for transfusion purposes23. However, despite these notable advancements, current European Council guidelines suggest that RBC concentrates may be stored for up to 42 days under controlled conditions before transfusion24.
It is now widely accepted that storage affects a wide array of biochemical and biological properties of RBCs to a significant extent, a phenomenon which goes by the name of storage lesions. Storage lesions include (i) alterations to RBC morphology (shape changes leading from a discoid to a spherocytic phenotype) or (ii) RBC functionality (metabolism and oxygen delivery capacity, through an increase in oxygen affinity mediated by a rapid fall in 2,3-diphosphoglycerate concentrations), as it has been recently reviewed7,14,16.
While some changes are reversible to some extent, such as restoring of 2,3-DPG reservoirs after transfusion26, others are not, especially those targeting the protein compartment (i.e. the proteome). Under a biochemical point of view, these irreversible changes to the proteome are best visualized when considering the variation of 2-dimensional electrophoretic (2DE) patterns of long-stored RBCs, which change dramatically as storage progresses with the presence of new spots in the RBC membrane protein profile8.
Since RBCs are devoid of any new protein synthesis capacity, these newly appearing spots are represented by:
Cytosolic proteins which are relocated at the membrane level as storage progresses;
Protein fragments in the low molecular weight (MW) range;
Protein aggregates in the high MW range.
These three categories of newly appearing protein spots either represent protein fragments, aggregates or cytosolic proteins relocating to the membrane (yet by storage day 218,17,18).
The first category of newly appearing spots might represent a realistic marker for blood doping upon transfusion, through direct targeted analyses. In a set of recent investigations17,18, we performed detailed analyses of RBC membrane protein changes to determine whether some of the cytosolic proteins relocating at the membrane could represent a suitable age-dependent biomarker of long-stored RBCs and their oxidation level. One of these relevant biomarkers was identified as the oxidative stress-related protein peroxiredoxin 2 (Prdx2)17,18.
In RBCs from healthy individuals, Prdx2 is normally located in the cytosol and thus western blot analyses toward Prdx2 in RBC membranes from day 0 units do not show Prdx2 immunoreactivity17. On the other hand, older units display immunopositivity for Prdx2 at the membrane17. On this basis, we concluded that, immunopositivity for Prdx2 in RBC membranes could be also a biomarker of RBC transfusion, as long as RBCs have been stored at least longer than three weeks (which is actually the case of transfusing athletes). Indeed, membranes of freshly withdrawn RBCs should be Prdx2-free, unless the athlete does not suffer from hereditary anomalies such as hereditary spherocitosis26, which is very infrequent (the incidence of hereditary spherocytosis is about 200 to 300 per million in northern European populations27) and also unlikely for professional athletes for obvious reasons.
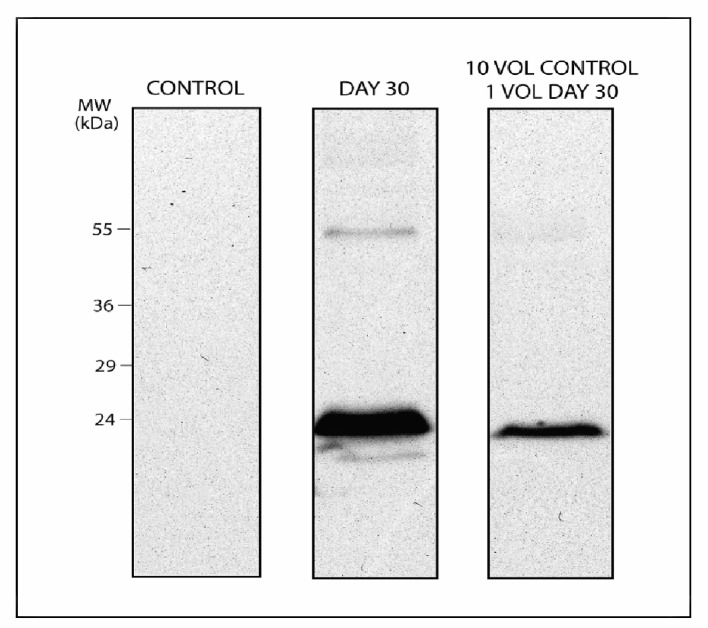
To understand whether this marker could be suitable for blood doping testing, we designed an in vitro model for autologous blood transfusion through diluting in a 1:10 ratio long-stored packed RBCs into freshly withdrawn concentrated RBCs (day 0 from the same donor) (the rationale behind this dilution ratio is further explained in the Methodologies section, and stems from a rough estimation of the actual dilution of transfused RBCs in the bloodstream of a healthy recipient, such as in the case of an athlete undergoing an autologous transfusion). This model has been thought as to mimic autologous transfusion in transfusing athletes through maintaining a 1:10 approximate ratio which is obtained upon transfusion of a whole unit of refrigerated packed RBCs in an adult male (5 liters of whole blood, hematocrit 45%). We chose 30 day-stored RBC as a long-stored RBC counterpart, which should represent a realistic model for athletes collecting blood at least one month and a half before a competition and transfusing ten days before. Through western blot against Prdx2 we could identify immunopositivity in RBC membranes from 30 day-stored concentrates while not in day 0 counterparts (Figure 1). Immunopositivity was still visible in the 1:10 dilution (though with a reduced intensity, see Figure 1).
Figure 1.
Peroxiredoxin 2 levels in day 0, day 30 and 1:10 dilution of day 30 in day 0 membrane protein extracts from RBC concentrates. Although less intensely, Prdx2 immunopositivity could be observed also upon dilution (right lane) under non-reducing conditions with two bands corresponding to the monomer and the dimer.
Using fluctuations in RBC population as an indicator of blood transfusion
While it has been evidenced the linkage between the rEPO-induced modulation of erythropoiesis and the alteration in the RBC population pattern (increased reticulocyte/mature RBC ratio)11, it has been so far only partially demonstrated that transfusion of long-stored RBCs (either stored hypothermically or cryostored) would result in anomalously biasing of the physiological percentage distribution in RBC subpopulations28.
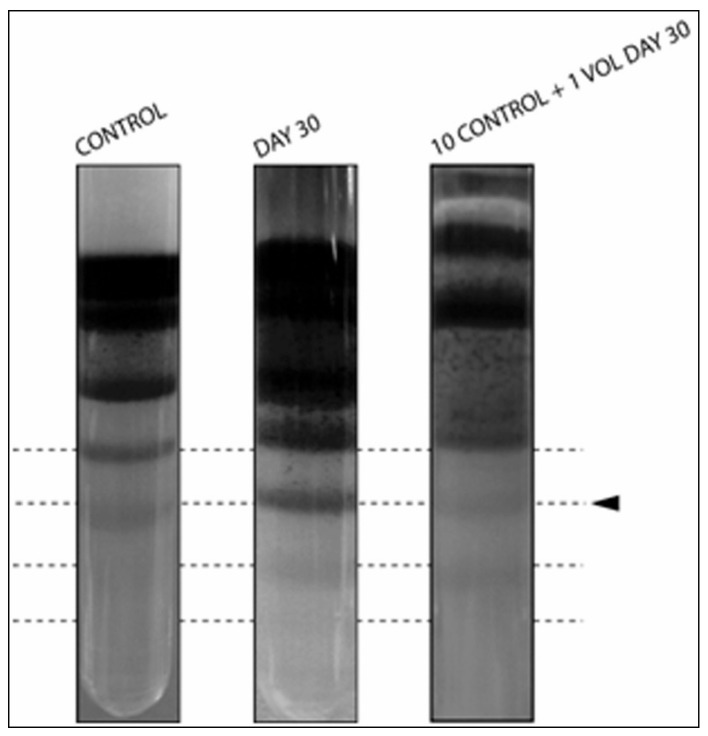
To this end, we conducted preliminary investigations with discontinuous density gradients to understand whether this approach might reveal precious insights for further development of anti-doping strategies. In particular, long stored RBCs (day 30: again, as a realistic model for an athlete auto-transfusing one week before the beginning of a competition a unit withdrawn one month and a half before) were mixed in a dilution 1:10 with freshly drawn (day 0 from the same donor) erythrocyte concentrates. As a result (Figure 2), newly detectable higher density bands (as older RBCs are also denser29) at day 30 appeared, which could still be visible upon dilution in 1:10 (day 30: day 0 RBCs).
Figure 2.
Density gradient of day 0 (left column), day 30 (right column) and 10 (day 0):1 (day 30) mixed RBC concentrates (center). The intensity from band 6 down (from top to bottom) increases in day 30 hypothermically stored RBCs and are still evident upon 10:1 mixing.
Conclusion
These preliminary results suggest for further experimenting in this direction, with the planning of larger scale in vivo studies to understand the robustness of the reported phenomena (Prdx2 membrane-immunopositivity and alteration of RBC populations upon transfusion of RBC concentrates which had been stored for longer than four weeks). Full automation of the RBC population analysis might be provided by the application of hemocromocitometric analyses on blood samples collected from transfused recipients. If results from further studies will confirm our preliminary results (Figure 1–2), it will be also necessary to monitor the kinetics of these changes in vivo (it is indeed difficult to postulate a priori how long would the membrane Prdx2 marker be still visible in RBC samples from recipients transfused with RBC stored longer than four weeks). This additional information would be pivotal to assess whether the Prdx2 biomarker would be detectable for a period long enough to allow for blood testing as a suitable anti-doping strategy.
Acknowledgments
The authors are supported by funds from the Italian National Blood Center (Centro Nazionale Sangue -CNS - Istituto Superiore Sanità - Rome, Italy).
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Segura J, Monfort N, Ventura R. Detection methods for autologous blood doping. Drug Test Anal. 2012 doi: 10.1002/dta.405. [DOI] [PubMed] [Google Scholar]
- 2.Lippi G, Banfi G. Blood transfusions in athletes. Old dogmas, new tricks. Clin Chem Lab Med. 2006;44(12):1395–402. doi: 10.1515/CCLM.2006.262. [DOI] [PubMed] [Google Scholar]
- 3.Giraud S, Sottas PE, Robinson N, Saugy M. Blood transfusion in sports. Handb Exp Pharmacol. 2010;195:295–304. doi: 10.1007/978-3-540-79088-4_13. [DOI] [PubMed] [Google Scholar]
- 4.Ashenden M. A strategy to deter blood doping in sport. Haematologica. 2002;87:225–34. [PubMed] [Google Scholar]
- 5.Baumann GP. Growth Hormone Doping in Sports: A Critical Review of Use and Detection Strategies. Endocr Rev. 2012 doi: 10.1210/er.2011-1035. [DOI] [PubMed] [Google Scholar]
- 6.Arndt PA, Kumpel BM. Blood doping in athletes--detection of allogeneic blood transfusions by flow cytofluorometry. Am J Hematol. 2008;83(8):657–67. doi: 10.1002/ajh.21196. [DOI] [PubMed] [Google Scholar]
- 7.D’Alessandro A, Liumbruno G, Grazzini G, Zolla L. Red blood cell storage: the story so far. Blood Transfus. 2010 Apr;8(2):82–8. doi: 10.2450/2009.0122-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocytefiltered red bood cell concentrates: from metabolism to proteomics. Haematologica. 2012;97(1):107–15. doi: 10.3324/haematol.2011.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pallotta V, D’Amici GM, D’Alessandro A, et al. Red blood cell processing for cryopreservation: from fresh blood to deglycerolization. Blood Cells Mol Dis. 2012 doi: 10.1016/j.bcmd.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Eichner ER. Blood doping: infusions, erythropoietin and artificial blood. Sports Med. 2007;37(4–5):389–91. doi: 10.2165/00007256-200737040-00030. [DOI] [PubMed] [Google Scholar]
- 11.Tsitsimpikou C, Kouretas D, Tsarouhas K, et al. Applications and biomonitoring issues of recombinant erythropoietins for doping control. Ther Drug Monit. 2011;33(1):3–13. doi: 10.1097/FTD.0b013e31820032c4. [DOI] [PubMed] [Google Scholar]
- 12.Ashenden M, Mørkeberg J. Net haemoglobin increase from reinfusion of refrigerated vs. frozen red blood cells after autologous blood transfusions. Vox Sang. 2011;101(4):320–6. doi: 10.1111/j.1423-0410.2011.01493.x. [DOI] [PubMed] [Google Scholar]
- 13.Solymos E, Guddat S, Geyer H, et al. Di(2-ethylhexyl) phthalate metabolites as markers for blood transfusion in doping control: intra-individual variability of urinary concentrations. Drug Test Anal. 2011;3(11–12):892–5. doi: 10.1002/dta.377. [DOI] [PubMed] [Google Scholar]
- 14.Lion N, Crettaz D, Rubin O, Tissot JD. Stored red blood cells: a changing universe waiting for its map(s) J Proteomics. 2010;73(3):374–85. doi: 10.1016/j.jprot.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Blasi B, D’Alessandro A, Ramundo N, Zolla L. Red blood cell storage and cell morphology. Transfus Med. 2012 Apr;22(2):90–6. doi: 10.1111/j.1365-3148.2012.01139.x. [DOI] [PubMed] [Google Scholar]
- 16.Bosman GJ, Werre JM, Willekens FL, Novotný VM. Erythrocyte ageing in vivo and in vitro: structural aspects and implications for transfusion. Transfus Med. 2008;18(6):335–47. doi: 10.1111/j.1365-3148.2008.00892.x. [DOI] [PubMed] [Google Scholar]
- 17.Rinalducci S, D’Amici GM, Blasi B, et al. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion. 2011;51(7):1439–49. doi: 10.1111/j.1537-2995.2010.03032.x. [DOI] [PubMed] [Google Scholar]
- 18.Rinalducci S, D’Amici GM, Blasi B, Zolla L. Oxidative stress-dependent oligomeric status of erythrocyte peroxiredoxin II (PrdxII) during storage under standard blood banking conditions. Biochimie. 2011;93:845–85. doi: 10.1016/j.biochi.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Olivieri E, Herbert B, Righetti PG. The effect of protease inhibitors on the two-dimensional electrophoresis pattern of red blood cell membranes. Electrophoresis. 2001;22(3):560–5. doi: 10.1002/1522-2683(200102)22:3<560::AID-ELPS560>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 20.Low FM, Hampton MB, Peskin AV, Winterbourn CC. Peroxiredoxin 2 functions as a noncatalytic scavenger of low-level hydrogen peroxide in the erythrocyte. Blood. 2007;109(6):2611–7. doi: 10.1182/blood-2006-09-048728. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Bosch FH, Werre JM, Roerdinkholder-Stoelwinder B, et al. Characteristics of red blood cell populations fractionated with a combination of counterflow centrifugation and Percoll separation. Blood. 1992;79(1):254–60. [PubMed] [Google Scholar]
- 23.Hess JR. An update on solutions for red cell storage. Vox Sanguinis. 2006;91:13–9. doi: 10.1111/j.1423-0410.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- 24.Council of Europe. Guide to the Preparation, Use and Quality Assurance of Blood Components. 16th edition. Strasbourg, France: European Directorate for the Quality of Medicines & HealthCare; 2011. Recommendation No. R (95) 15. [Google Scholar]
- 25.Valeri CR, Collins FB. Physiologic effects of 2,3-DPG-depleted red cells with high affinity for oxygen. J Appl Physiol. 1971;31:823–7. doi: 10.1152/jappl.1971.31.6.823. [DOI] [PubMed] [Google Scholar]
- 26.Rocha S, Vitorino RM, Lemos-Amado FM, et al. Presence of cytosolic peroxiredoxin 2 in the erythrocyte membrane of patients with hereditary spherocytosis. Blood Cells Mol Dis. 2008;41(1):5–9. doi: 10.1016/j.bcmd.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Eber SW, Pekrun A, Neufeldt A, Schröter W. Prevalence of increased osmotic fragility of erythrocytes in German blood donors: screening using a modified glycerol lysis test. Ann Hematol. 1992;64:88. doi: 10.1007/BF01715351. [DOI] [PubMed] [Google Scholar]
- 28.Pottgiesser T, Sottas PE, Echteler T, et al. Detection of autologous blood doping with adaptively evaluated biomarkers of doping: a longitudinal blinded study. Transfusion. 2011;51(8):1707–15. doi: 10.1111/j.1537-2995.2011.03076.x. [DOI] [PubMed] [Google Scholar]
- 29.D’Alessandro A, Blasi B, D’Amici GM, et al. Red blood cell populations in freshly drawn blood: application of proteomics and metabolomics to a decades-long biological issue. Blood Transfusion. 2012 doi: 10.2450/2012.0164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]