Fig. 1. CD25 and CTLA-4 blockade: complementary strategies to overcome regulatory T-cell immunosuppression.
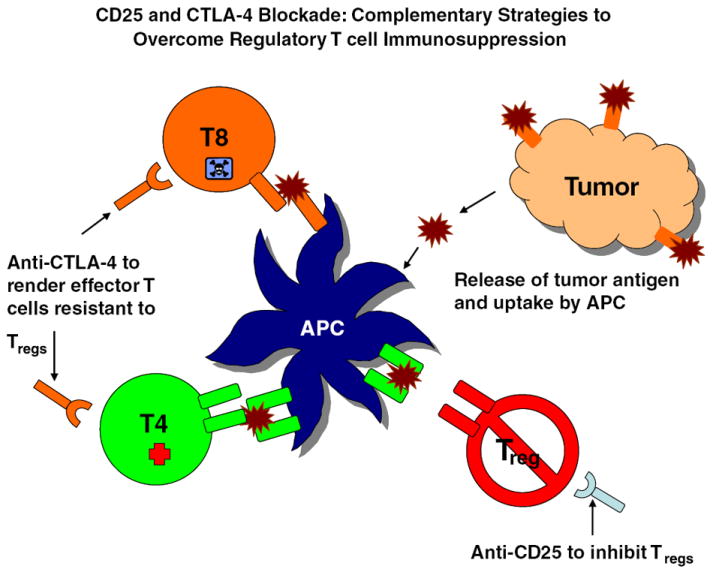
Systemic CTLA-4 blockade in experimental mice enhances CD4+CD25− T-cell proliferation and makes them resistant to Treg-mediated suppression but does not alter Treg function (373). Systemic anti-CD25 administration only partially depletes Tregs but renders remaining Tregs incapable of mediating T-cell suppression (142). Thus, CD25 and CTLA-4 blockade represent potentially complementary strategies for overcoming Treg-mediated immunosuppression in patients with malignant glioma. The diagram shows potential synergistic effects of anti-CTLA-4 and anti-CD25 mAb treatment in enhancing activation of tumor-specific lymphocytes. Antigen released by dying tumor cells is taken up and processed by resident or infiltrating APCs and presented as peptides to CD4+ and CD8+ T cells. Tregs, which are elevated in proportion in patients with malignant glioma, attenuate these responses through interaction with APCs and T cells, and these suppressive effects may be counteracted through anti-CD25 treatment to partially deplete Tregs and functionally inactivate remaining Tregs as well as through CTLA-4 blockade, which renders activated effector cells resistant to Treg-mediated immunosuppression.
