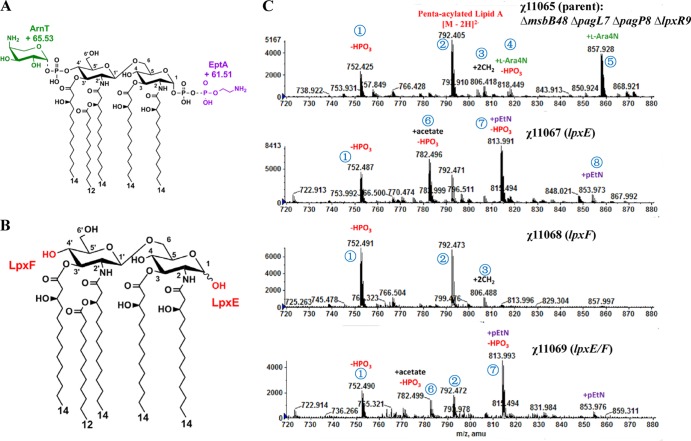
Fig 1.
Lipid A structure of the S. Typhimurium msbB mutant and its derivatives. (A) Covalent modifications of lipid A in the msbB pagL pagP lpxR mutant. The known covalent modifications of lipid A are indicated. (B) Covalent modifications of lipid A in the msbB pagL pagP lpxR mutant in the presence of LpxE or LpxF. (C) Lipid A profiles from ESI-MS analysis of χ11065 (ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR9), χ11067 (ΔmsbB48 ΔpagL7 ΔpagP81::Plpp lpxE ΔlpxR9), χ11068 (ΔmsbB48 ΔpagL7 ΔpagP8 ΔlpxR93::Plpp lpxF), and χ11069 (ΔmsbB48 ΔpagL7 ΔpagP81::Plpp lpxE ΔlpxR93::Plpp lpxF) grown in LB medium at 37°C. The msbB pagL pagP lpxR mutant makes a penta-acylated lipid A as indicated by the [M − 2H]2− peak at m/z 792.55. Removal of one phosphate group shifts the lipid A [M − 2H]2− peak by m/z −39.98. The addition of l-Ara4N to the 4′ phosphate, catalyzed by ArnT, shifts the lipid A [M − 2H]2− peak by m/z +65.529. The addition of pEtN to the 1-phosphate, catalyzed by EptA, shifts the MS peak by m/z +61.505. See Table S3 in the supplemental material for detailed information on lipid A derivatives.