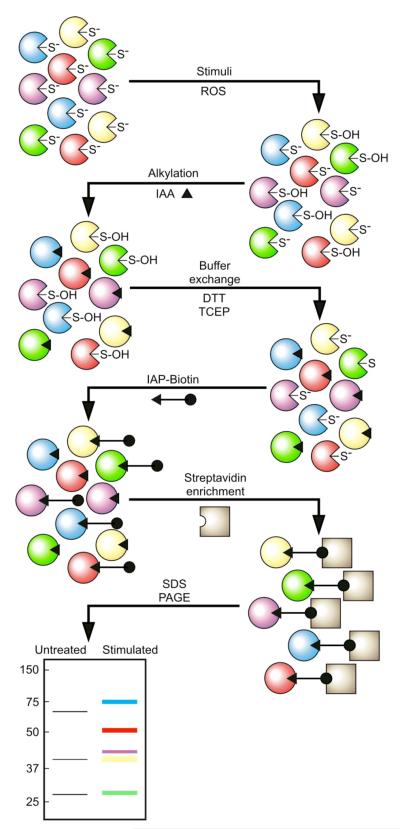
Fig. 1.
Profiling of reversible PTP oxidation. The catalytic Cys residue of members of the PTP family of enzymes is present as a thiolate under basal cellular conditions. This feature makes it a good nucleophile at neutral pH and allows it to react with phospho–amino acid residues, ROS, or certain sulhydryl-reactive compounds. After a stimulus that generates localized production of ROS, the active-site Cys residue of those specific PTPs that encounter the ROS becomes oxidized, and PTP activity is abrogated. The pool of reversibly oxidized PTPs is represented by a sulfenic acid (S-OH). In the first step of the assay, the active-site Cys residue of those PTPs that were not modified by second messenger ROS molecules in vivo is modified irreversibly by alkylation under anaerobic conditions in vitro. In the second step, the active-site Cys residues of PTPs that were reversibly oxidized and thus protected from alkylation in the first step are reduced. DTT indicates dithiothreitol and TCEP indicates tris(2-carboxyethyl)phosphine. The third step consists of a modification in which the reduced Cys residue is modified by a biotinylated sulfhydryl-reactive probe under low-pH labeling conditions. Probe-labeled PTPs are then captured on streptavidin beads, enriched, and subjected to SDS-PAGE protein fractionation, transferred onto a nitrocellulose membrane, and detected with an antibody against biotin or a PTP-specific antibody.