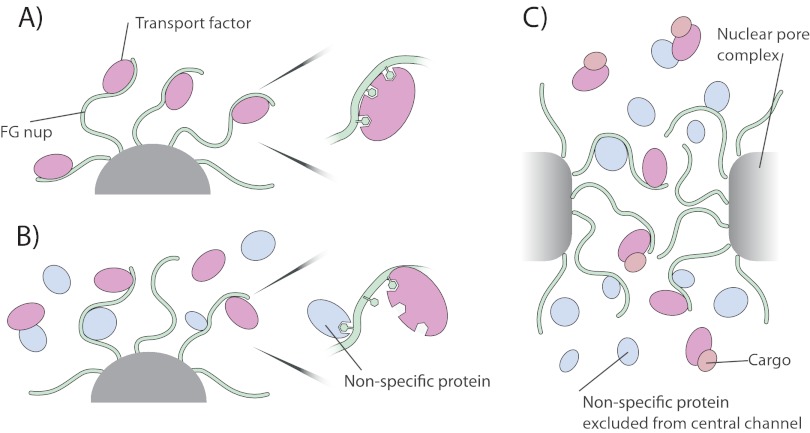
Fig. 10.
Potential implications of our results for nucleocytoplasmic transport. A, in the absence of monovalent competitors, Kaps bind tightly to the FG Nups, preventing rapid exchange. B, however, monovalent competitor reduces the valency and so avidity of Kaps for FG Nups, allowing a rapid and dynamic exchange of the two (a zoomed example of competitor interacting with FG repeats is shown; competitor interaction with Kap is also possible). C, within the nuclear pore complex, Kaps and FG nups are in contact with other FG nups, transport factors, cargo, and small proteins that can rapidly diffuse through the central channel. All of these factors contribute to rapid and effective transport of Kap/cargo complexes through the NPC and serve to block the passage of non-Kap-bound macromolecules.