Abstract
Hydroxylated fullerenes (C60OHx) or fullerols are water-soluble carbon nanoparticles that have been explored for potential therapeutic applications. This study assessed acute in vivo tolerance in 8 week old female Sprague Dawley rats to intravenous administration (IV) of 10 mg/kg of well-characterized C60(OH)30. Complete histopathology and clinical chemistries were assessed at 8, 24, and 48 hr after dosing. Minor histopathology changes were seen, primarily in one animal. No clinically significant chemistry changes were observed after treatment. These experiments suggest that this fullerol was well tolerated after IV administration to rats.
INTRODUCTION
Hydroxylated fullerenes (fullerols) are water-soluble carbon nanoparticles (NP) which may hold some promising medical applications (Dordević and Bogdanović, 2008). Recent in vitro studies in our lab demonstrated minimum cytotoxicity when exposed to human epidermal keratinocytes at three levels of hydroxylation (C60OHX, X = 20, 24, 32) and at relatively high exposure concentrations (Saathoff et al., 2011). Functionalization of fullerenes, such as by hydroxylation, increases water solubility and was reported to reduce oxidative stress and some forms of cytotoxicity to cells and tissues (Sayes et al., 2004; Bogdanovć et al., 2004; Dordević and Bogdanović , 2008; Injac et al., 2008; Xu et al., 2010). Some studies showed cytotoxicity at non-environmentally relevant exaggerated in vitro concentrations and embryo toxicity in zebrafish (Jovanović et al., 2010). A review of the biodistribution and pharmacokinetics of carbon nanomaterials in mice and rabbits suggests distribution primarily to reticuloendothelial cells and organs (e.g., liver, spleen, and thymus) and some elimination in the urine, a route not seen with the more lipophilic non-functionalized native fullerenes (Almeida et al., 2010, Riviere, 2009, Rajagopalan et al., 1996). Uptake of water soluble fullerenes to normal tissues may be minimal as suggested for nC60 in perfused skin studies (Leavens et al., 2010).
There have been few studies using well characterized materials to assess in vivo multi-organ pathology or clinical chemistry after administration of a single dose of hydroxylated fullerenes to a common lab animal model such as the rat. Rat studies conducted to date focused solely on NP distribution to blood and organs without assessment of damage to these tissues. This is understandable since pharmacokinetic and biodistribution studies are usually destructive for mass balance endpoints and do not assess multi-organ pathology. The objective of the present study was to comprehensively assess acute biological effects after a single 10mg/kg IV dose of a fully characterized hydroxyfullerene (C60(OH)30) to female Sprague-Dawley rats.
METHODS
Preparation of Hydroxylated Fullerenes
Hydroxylated fullerenes were prepared using the phase transfer method of Li et al. (1993). Sodium hydroxide (NaOH) was mixed with a solution of C60 (SES Research, Houston, TX) and toluene, then added to the phase transfer agent (40% aqueous tetrabutylammonium hydroxide (TBAH)). The purple solution was agitated for 30 min to form a colorless solution with a brown precipitate. The toluene phase was aspirated and residual toluene removed by evaporation under vacuum. Deionized water was then added to the product, and the solution mixed for 24 hr with constant air flow to solubilize the precipitate and allow hydroxylation of the product. Excess CO2 was removed through a 1 M NaOH column and the product washed thrice with methanol to remove residual NaOH and TBAH. The C60(OH)x product was solubilized in methanol, centrifuged at 1450 xg for 10 min, and supernatant removed to recover the product. Subsequent washes in 10 ml deionized water dissolved the product, with methanol added to precipitate the product for centrifugation. In the final step, the product was dissolved in water, passed through a 0.2 μm nylon filter, and dried under vacuum. Further hydroxylation was promoted by adding 3% hydrogen peroxide (H2O2) after the final methanol treatment. The precipitate was dissolved in 25 ml 3% H2O2 and stirred in a sealed bottle for 24 hr. Washing and drying steps performed for the H2O2 treated product were the same as above.
Characterization of Hydroxylated Fullerenes
The degree of fullerene hydroxylation was measured via x-ray photoelectron spectroscopy (XPS) using a Kratos Axis ULTRA system (Shimadzu). The fullerol powder was collected to a thickness greater than 500 μm on double-stick copper tape, with survey and high-resolution scans performed (scan area: 300 μm x 700 μm) to obtain elemental composition and functionality. The spectra were obtained using a pass energy of 160 eV (step size 1 eV), and high-resolution scans were generated with a pass energy of 20 eV (step size 0.1 eV). Binding energies were standardized using the C 1s peak (284.6 eV). The number of hydroxyl groups for the C60 derivative was estimated using the peak areas of high-resolution scans of C 1s and O 1s, with the results averaged from three sample spectra. The results were confirmed using attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR). The fullerenol powder was deposited on a germanium crystal, and the spectra collected with a Thermo Electron Nicolet 8700 ATR-FTIR spectrometer at a 4 cm−1 resolution with 256 scans. Background spectra were generated with the blank Ge crystal prior to running the sample. A C60(OH)24 sample (MER Corp, AZ) was used as the standard reference material to validate the XPS and ATR-FTIR results.
The NP size was measured by dynamic light scattering (DLS) at 22°C with a Zetasizer Nano ZS (Malvern Instruments) of a 1 mg/ml dilution of the fullerenol in deionized water. In addition, 10 μl stock fullerenol was placed onto a formvar-coated grid, air-dried overnight, and examined on a FEI/Philips EM208S transmission electron microscope operating at an accelerating voltage of 80 KV.
Animals
White, female, 8 week old Sprague-Dawley rats weighing between 200–250 g were obtained from Charles River Laboratories, Inc. (Morrisville, NC) and quarantined for 14 days to ensure they were healthy with no underlying medical conditions. Once removed from quarantine, rats were individually housed in Nalgene Metabolic Cages with a 150–300 g capacity (Nalgene, Rochester, NY) and allowed a one week acclimation period prior to the beginning the study. Animals, exposed to a normal diurnal cycle (12 hr light/dark cycle) with environmental controls maintained at approximately 23°C and 43–47% relative humidity, were treated in accordance with the guidelines prepared by the Institute of Laboratory Animal Resources (1996). The rats were watered and fed a ground diet of Purina Lab Diet 5001 (PMI, Richmond, IN) ad libitum.
Animal Dosing
The rats were divided into 4 groups: 8, 24, and 48 hr treatment (n=4/group) and 48 hr control (n=4). Blood, urine, and feces were collected from the treatment groups prior to dose. The C60(OH)30 dosing solution was prepared under sterile conditions by adding 7.5 ml sterile nonpyrogenic water (USP for injection) (Baxter Healthcare Co., Deerfield, IL) to 75 mg of the hydroxylated fullerene powder in a sterile, nonpyrogenic injection vial (Hospira, Lake Forest, IL). The 10 mg/ml solution was vortexed for 10 min to solubilize the product. The rats were immobilized in a Tailveiner®Rat Restraint System (Braintree Scientific, Inc.; Braintree, MA) with the tail exteriorized for access to the lateral tail veins. The injection site was cleaned with an alcohol pad and a 10 mg/kg IV dose of C60(OH)30 was administered via a lateral tail vein with a 25-gauge needle on a 1 ml syringe. The lateral tail vein of one animal in the 8 hr group collapsed during the injection, preventing a full dose of the fullerol to enter the bloodstream. This animal was removed from the study, leaving three animals in this group.
Sample Collection
Following administration of dose, blood and urine were collected from the rats at termination of dosing at 8 and 48 hr. The blood was centrifuged, plasma collected, and both samples stored at −80°C for later analysis. Urine collected at each sample time was placed into glass vials, quantitated, and stored at −80°C. After 8, 24, and 48 hr, each group of rats was euthanized via CO2 asphyxiation according to the AVMA Guidelines on Euthanasia (2007). Each animal was necropsied, with 29 target tissues collected and weighed. The tissues included liver (one sample from each of the 4 lobes), kidneys, urinary bladder, thymus, lymph nodes (3 samples – mesenteric, subcutaneous and inguinal), brain, adrenal glands, heart, muscle (2 samples– quadriceps and gluteals), full thickness skin (dorsal and ventral sites), adipose tissue (subcutaneous and intra-abdominal), ovaries, stomach, small intestines (duodenum, jejunum and ileum), pancreas, colon (descending), lungs, spleen and skin surrounding the injection site. The tissues were trimmed and fixed in 10% neutral-buffered formalin. The remaining unfixed tissue was weighed and stored at −80°C.
Histopathology
All tissue samples were processed routinely through graded alcohols, cleared in Clearite, and infiltrated and embedded paraffin in a Tissue-Tek VIP 2000 Tissue Processor (Miles Scientific). Tissue samples were sectioned at 5 μm, mounted on glass slides, stained with hematoxylin and eosin (H&E), and cover-slipped with PermountR. Slides were randomized by tissue type using a random number generator and evaluated blinded for any histopathology changes. All tissue changes were graded on a standard severity scale of minimal, mild, moderate or severe.
Blood and Urine Chemistry
Blood chemistry was performed with a COBAS Integra 400 Plus System chemical analyzer (Roche Diagnostics Corp., Indianapolis, IN) on plasma samples from the 48 hr treatment and control groups to determine chemical, endocrine, or electrolyte fluctuations. Blood chemistry parameters that were measured included blood urea nitrogen (BUN), creatinine, Na, K, Ca, Cl, P, total protein, albumin, globulin, cholesterol, total bilirubin, alkaline phosphatase (ALP), alanine aminotransferase (ALT), and gamma-glutamyl transpeptidase (GGT). Urine protein and creatinine levels were determined with the COBAS Integra 400 Plus System only for the 0 and 24 hr time points in the 24 and 48 hr treatment groups (n=8) to screen for potential renal dysfunction.
Statistical Analysis
The mean values for each treatment were calculated and significant differences (p < 0.05) determined using the Student’s t-test. DLS data was also analyzed for outlying data using analysis software integrated into the Malvern Zetasizer Nano system.
RESULTS
Particle Characterization
XPS analysis of the particle found 30 associated hydroxyl groups (-OH), confirmed by an FTIR measurement of 28.425 groups per particle, rendering a chemical formula of C60(OH)30. DLS measurements of the fullerol dose diluted to 1 mg/ml in deionized water and measured at 22°C resulted in a mean particle size of 267.98 nm diameter. Transmission electron microscopy revealed that the electron-dense NP in the stock solution was fairly homogenous in size with some agglomerates up to 50 nm in diameter (Figure 1).
Figure 1.
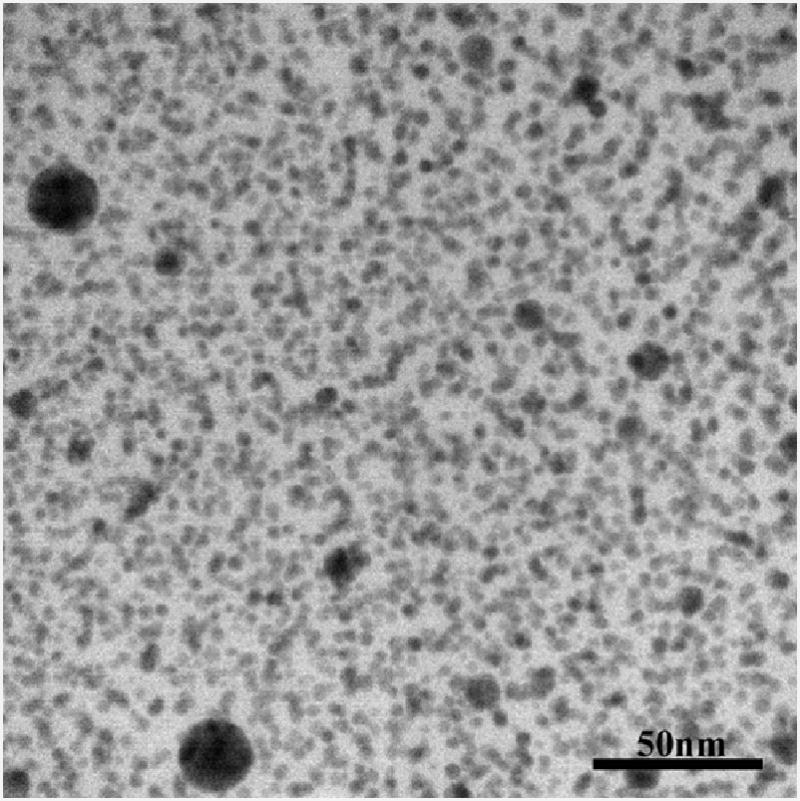
Transmission electron micrograph of stock C60(OH)30 fullerenol air-dried overnight on a formvar-coated grid.
Histopathology
While gross inspection of intact tissues during necropsy revealed no evident pathologic changes, some minor changes were noted during histopathology analysis. The 8 hr group (n=3) showed one incident of minimal perivascular lymphocyte infiltration in thymus tissue, one incident of minimal diffuse hepatocyte vacuolation and one incident of mild hepatic sinusoidal lining cell hypertrophy in a right liver lobe, one incident of minimal diffuse hepatocyte vacuolation in a middle liver lobe, one incident of minimal diffuse hepatocyte vacuolation in a left liver lobe, one incident of minimal diffuse hepatocyte vacuolation in a caudate liver lobe, two incidents of mild sinusoidal lining cell hypertrophy in two caudate liver lobes, and one incident of mild subdermal neutrophilic infiltration and one incident of moderate perivascular neutrophilic infiltration in the skin of the injection site in two rats.
Histopathology changes in the 24 hr group (n=4) include two incidents of minimal myocardial perivascular lymphocyte infiltration in two rats, one incident of mild sinusoidal lining cell hypertrophy in a caudate liver lobe, and one incident of minimal subdermal mononuclear infiltration and one incident of mild diffuse stromal cell hypertrophy in the skin of the injection site in two rats. Changes in the 48 hr group (n=4) showed one incident of mild neutrophilic infiltration in a cardiac valvular base, one incident of minimal lymphocytic infiltration in mesenteric adipose tissue, one incident of moderate cystitis with mixed neutrophilic/lymphoplasmacytic infiltration, edema and hemorrhage, one small hepatic granuloma in one focus of a left liver lobe, and multiple derangements evident in the skin of the injection site of one rat including focal dermal and subdermal neutrophil and macrophage infiltration, mild regional hypertrophy of stromal cells, regional subdermal mononuclear infiltration, focal dermal necrosis, and focal dermal hemorrhage.
Few histopathology changes were noted within the control group and were considered normal individual biological processes. Changes seen in treatment groups were not considered abnormal if the respective control group showed more than one instance of equivalent change.
Blood and Urine Chemistry
While a complete blood chemistry (CBC) analysis was performed on all 48 hr blood samples, only the parameters with renal or hepatic consequence were included in the final analysis. Several individual blood values fell outside of the range set by the controls, but statistical analysis found only phosphorus (P) and potassium (K) were significantly different from control. The means of the control group for P and K measured 7.7mg/dl and 6.05mM/ respectively, while the means for the treatment group was 8.68mg/dl for P and 7.85mM/l for K (Table 1). These are all within clinically normal values.
Table 1.
Blood chemistry values
| Control | Treated | Units | |
|---|---|---|---|
| Urea Nitrogen (BUN) | 17.0 ± 0.8 | 15.3 ± 2.3 | mg/dl |
| Creatinine (Creat) | 0.4 ± 0.0 | 0.4 ± 0.03 | mg/dl |
| Phosphorus (P) | 7.7 ± 0.2 | 8.7 ± 0.3* | mg/dl |
| Calcium (ca) | 13.4 ± 0.3 | 12.1 ± 0.6 | mg/dl |
| Total Protein (TP) | 7.1 ± 0.1 | 6.6 ± 0.3 | g/dl |
| Albumin (Alb) | 4.8 ± 0.1 | 4.2 ± 0.4 | g/dl |
| Globulin (Glob) | 2.4 ± 0.03 | 2.4 ± 0.1 | g/dl |
| Cholesterol (Chol) | 75.3 ± 10.1 | 68.8 ± 5.9 | mg/dl |
| Total Bilirubin (Tbili) | 0.03 ± 0.03 | 0.2 ± 0.1 | mg/dl |
| Alkaline Phosphatase (ALP) | 118.8 ± 20.5 | 135.8 ± 18.3 | IU/l |
| Alanine Aminotransferase (ALT) | 49.5 ± 5.3 | 52.5 ± 5.5 | IU/l |
| Gamma Glutamyl Transpeptidase (GGT) | 0.0 ± 0.0 | 0.0 ± 0.0 | IU/l |
| Sodium (Na) | 144.0 ± 0.6 | 144.8 ± 1.3 | mM/l |
| Potassium (K) | 6.1 ± 0.2 | 7.9 ± 0.3* | mM/l |
| Chloride (Cl) | 94.8 ± 0.8 | 100.5 ± 2.7 | mM/l |
significantly different than paired control (p <0.05)
Mean ± SEM of rats from the 48 hr treated and control groups (n=4/group).
Urine production was not consistent across all rats, with 8 rats producing urine within 2hr of the dose. Of the 8 rats, 6 rats produced dark urine, which indicated the presence of fullerols while the remaining two rats produced clear urine. All urine collected from this point forward was clear. The 24 hr urine protein and creatinine levels and subsequent protein/creatinine ratios (P/C) show a significant difference in the mean P/C ratio of 0.52 compared to the 0 hr mean ratio of 0.64 (Table 2). No statistical differences were found between the individual protein and creatinine levels.
Table 2.
Urine protein and creatinine values with Protein/Creatinine (P/C) ratio
| 0 hr | 24hr | |
|---|---|---|
| Protein | 32.9 ± 3.7 | 45.0 ± 6.6 |
| Creatinine | 62.5 ± 5.9 | 69.2 ± 9.3 |
| P/C ratio | 0.5 ± 0.03 | 0.6 ± 0.04* |
significantly different than paired control (p <0.05)
(Mean ± SEM) of 0 and 24 hr samples from the 24 and 48 hr treatment groups.
DISCUSSION
The histopathology observations represent those samples which differ significantly from control. Of the approximately 319 treated samples that were examined, only 18 samples showed any change with the potential to indicate a potentially toxic response to the treatments. These 18 samples encompassed 9 individual rats, with half of the potential inflammatory events occurring in only two of the treated rats (one at 8 hr and one at 48 hr) and these primarily related to the skin injection site, an area of known trauma due to the needle stick. Therefore, these results can not be attributed solely to the hydroxylated fullerene treatment.
Removing injection site data, two potential observations remain. The first relates to hepatotoxicity in an organ known for fullerenols accumulation (Riviere, 2009). Markers of inflammation or hepatocyte adaptation were noted in 7 different liver samples spread across all 4 lobes with 4 instances of multifocal sinusoidal cell hypertrophy, one granuloma, and 4 instances of hepatocyte vacuolation. Since the 4 vacuolation occurrences represent only one rat, this may be normal variation, isolated disease, or response to treatment. In addition, 5 of the liver events represent the 8 hr time point, but again 4 of these occur in the same rat. The second trend shows that perivascular infiltration of inflammatory cells occurs 4 times for the possibility of a low-level vascular response to treatment. Two occurred in the heart after 24 hr, with one in the thymus and one at the injection site (not necessarily explained by localized trauma) after 8 hr. All the remaining occurrences of potential consequence were isolated events in individual tissues. It is possible that hepatic sinusoidal cell hypertrophy and hepatocytic vacuolation represent a direct effect of treatment. However, half of the hepatic events occurred in a single animal so no trend would exist if another explanation is possible for these abnormalities. Clinical chemistry changes were not clinically significant.
Overall, based on histopathology, blood and urine clinical chemistry systemic exposure of C60(OH)30 to the rat for up to 48 hr produced negligible toxicity, confirming the short term safety of these NP to rodent biological systems.
Acknowledgments
This research was supported by the National Institutes of Health (NIH-RO1 ES016138).
Contributor Information
Nancy A. Monteiro-Riviere, Email: Nancy_Monteiro@ncsu.edu.
Keith E. Linder, Email: Keith_Linder@ncsu.edu.
Alfred O. Inman, Email: Al_Inman@ncsu.edu.
John G. Saathoff, Email: John_Saathoff@ncsu.edu.
Xin-Rui Xia, Email: Summer_Xia@ncsu.edu.
Jim E. Riviere, Email: Jim_Riviere@ncsu.edu.
References
- Almeida JPM, Chen AL, Foster A, Drezek R. In vivo biodistribution of nanoparticles. Nanomedicine. 2010;6:815–835. doi: 10.2217/nnm.11.79. [DOI] [PubMed] [Google Scholar]
- American Veterinary Medical Association (AVMA) Guidelines on Euthanasia. 2007 Jun;:1–39. http://www.avma.org/issues/animal_welfare/euthanasia.pdf.
- Bogdanović G, Kojić V, Dordević A, Canadanović-Brunet J, Vojinović-Miloradov M, Baltić VV. Modulating activity of fullerol C60(OH)22 on doxorubicin induced cytotoxicity. Toxicol in Vitro. 2004;18:629–637. doi: 10.1016/j.tiv.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Dordević A, Bogdanović G. Fullerenol: a new nanopharmaceutic? Arch Oncol. 2008;16:42–45. [Google Scholar]
- Injac R, Boskovic M, Perse M, Koprivec-Furlan E, Cerar A, Djordjevic A, Strukelj B. Acute doxorubicin nephrotoxicity in rats with malignant neoplasm can be successfully treated with fullerenol C60(OH)24 via suppression of oxidative stress. Pharmacol Rep. 2008;60:742–749. [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the Care and Use of Laboratory Animals. National Academies Press; Washington, DC: 1996. [DOI] [PubMed] [Google Scholar]
- Jovanović B, Anastasova L, Rowe E, Palić D. Hydroxylated fullerenes inhibit neutrophil function in fathead minnow (Pimephales promelas Rafinesque, 1820) Aquat Toxicol. 2011;101:474–482. doi: 10.1016/j.aquatox.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Leavens TL, Xia XR, Lee HA, Monteiro-Riviere NA, Brooks JD, Riviere JE. Evaluation of perfused porcine skin as a model system to quantitate tissue distribution of fullerene nanoparticles. Toxicol Lett. 2010;197:1–6. doi: 10.1016/j.toxlet.2010.03.1119. [DOI] [PubMed] [Google Scholar]
- Li J, Takeuchi A, Ozawa M, Xinhai Li XH, Saigob K, Kitazawaa K. C60 fullerol formation catalysed by quaternary ammonium hydroxides. J Chem Soc Chem Commun. 1993;23:1784–1785. [Google Scholar]
- Rajagopalan P, Wudl F, Schinazi RF, Boudinot FD. Pharmacokinetics of a water soluble fullerene in rats. Antimicrob Agents Chemother. 1996;40:2262–2265. doi: 10.1128/aac.40.10.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere JE. Pharmacokinetics of nanomaterials: an overview of carbon nanotubes, fullerenes and quantum dots. WIRES Nanomed Nanobiotechnol. 2009;1:26–34. doi: 10.1002/wnan.24. [DOI] [PubMed] [Google Scholar]
- Saathoff JG, Inman AO, Xia XR, Riviere JE, Monteiro-Riviere NA. In vitro toxicity assessment of three hydroxylated fullerenes inhuman skin cells. Toxicol in Vitro. 2011;25:2105–1221. doi: 10.1016/j.tiv.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayes CM, Fortner JD, Guo W, Lyon D, Boyd AM, Ausman KD, Tao YJ, Sitharaman B, Wilson LJ, Hughes JB, West JL, Colvin VL. The differential cytotoxicity of water-soluble fullerenes. Nano Lett. 2004;4:1881–1887. [Google Scholar]
- Xu JY, Su YY, Cheng JS, Li SX, Liu R, Li WX, Xu GT, Li QN. Protective effects of fullerenol on carbon tetrachloride-induced acute hepatotoxicity and nephrotoxicity in rats. Carbon. 2010;48:1338–1396. [Google Scholar]


