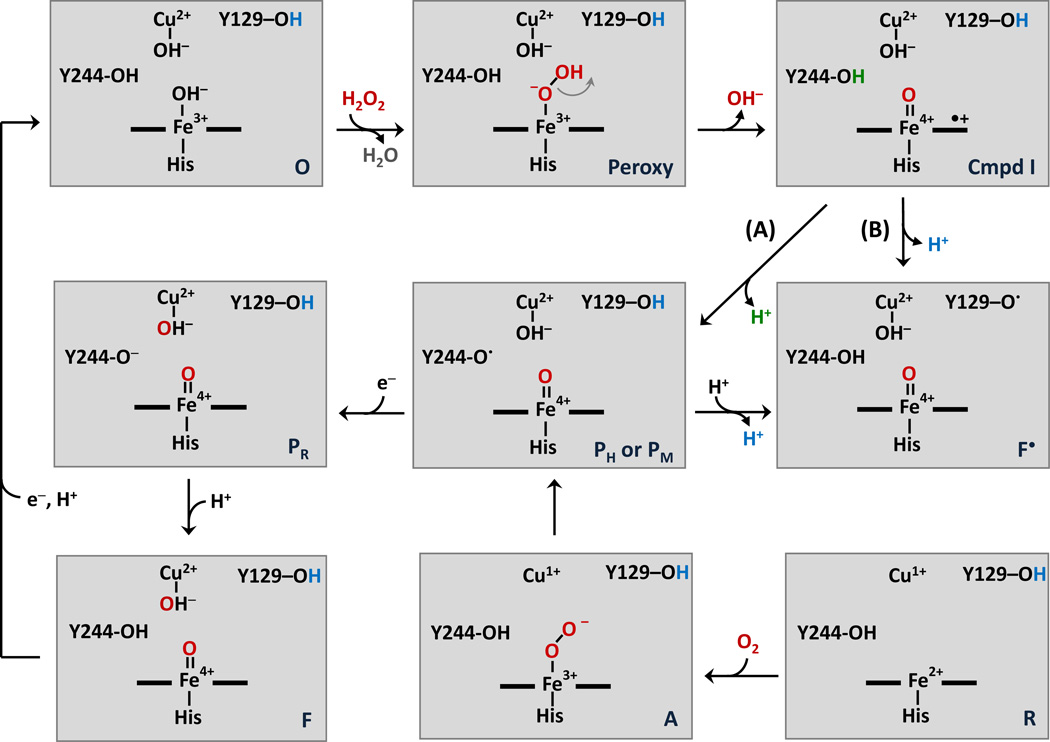
Figure 1. Postulated mechanisms for the reaction of oxygen with the reduced CuB-heme a3 binuclear center in CcO and for the reaction of H2O2 with the oxidized enzyme.
In this scheme the reaction with O2 with the reduced binuclear center starts from the lower left corner and progresses to the PM intermediate shown in the center. PM is associated with a radical on Y244, which donates an electron to the binuclear center following the O-O bond cleavage reaction. The reaction sequence progresses to the PR and F intermediates and ultimately to the oxidized state on the top left. The reaction with H2O2 is initiated by its binding to the heme a3 of the oxidized enzyme. The oxidized bCcO preparation used for the present work contains a peroxide bridged between Cu and Fe.25, 56 Thus, the O species in the scheme are likely to be formed by the H2O2 reduction of the bridged peroxide. The O-O bond cleavage of the resulting hydroperoxy intermediate leads to a Compound I species with a porphyrin π-cation, which can accept an electron from Y244 to form PH, leaving a radical on Y244 (Pathway A). The Y244 radical can be re-reduced by Y129 to form F•. Alternatively, the porphyrin π-cation can accept an electron directly from Y129 to form F• without passing through Y244 (Pathway B). The PH intermediate formed via Pathway A is the same as that (PM) generated in the O2 reaction and depending on the pH can progress to form PR and F.