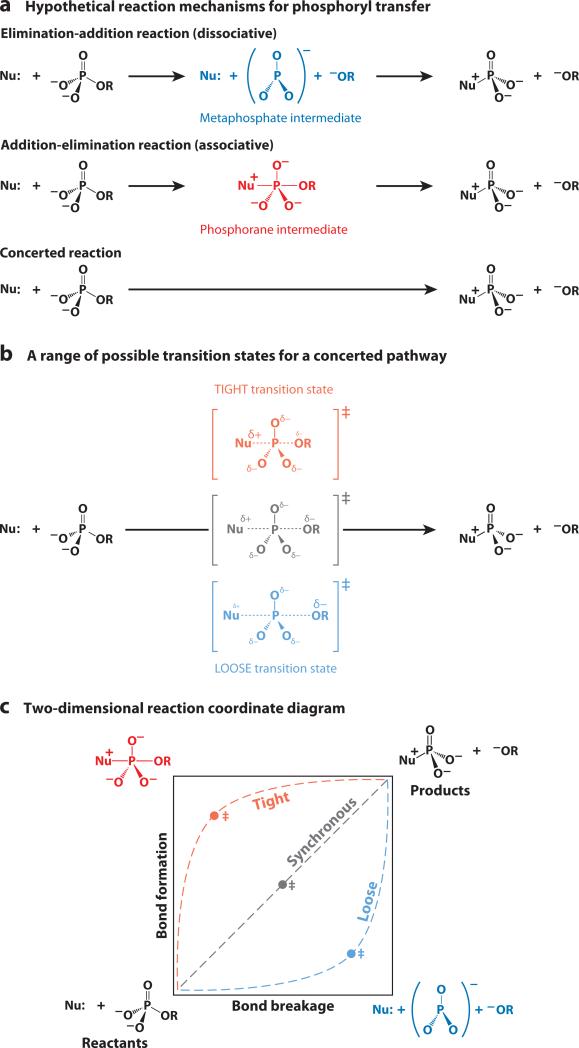
Figure 3.
Mechanistic possibilities in phosphoryl-transfer reactions. (a) An elimination-addition reaction through a metaphosphate intermediate (DN + AN), an addition-elimination reaction through a phosphorane intermediate (AN + DN), and a concerted reaction pathway with simultaneous bond formation and bond cleavage (ANDN). The parentheses give the IUPAC nomenclature (162). (b) A range of possible transition states for a concerted pathway. (c) A two-dimensional reaction coordinate diagram, also known as a More O'Ferrall-Jencks diagram (23, 24). The diagram represents a range of possible concerted reaction pathways passing through loose, synchronous, or tight transition states. Bond breaking and bond formation proceed along the x- and y-axes, respectively, and the energy axis is perpendicular to the page. The transition state is located at a maximum along the path from reactants to products, but a minimum in the direction perpendicular to the reaction pathway. The two-dimensional reaction coordinate depiction emphasizes that there is a continuum of reaction pathways, and the transition state can occur at any point along the pathway. For simplicity, the diagram above depicts symmetrical transition states halfway along each reaction pathway. Reactions proceeding through intermediates would have additional energy wells in the lower right corner for a metaphosphate intermediate and in the upper left corner for a phosphorane intermediate.