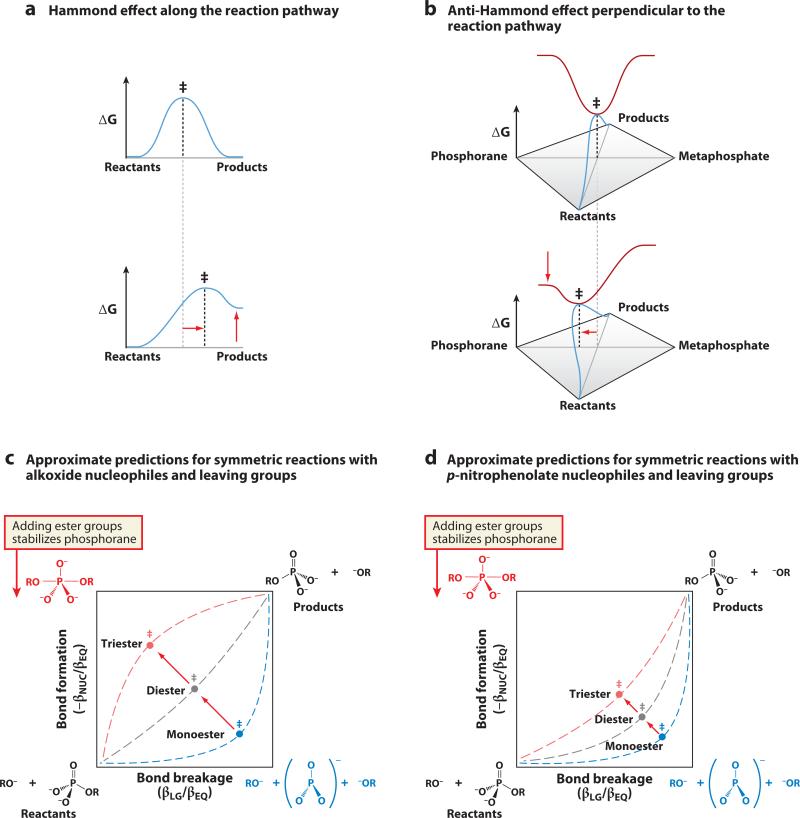
Figure 6.
Changes in structure can alter the nature of the transition state. (a) Along the reaction pathway, the transition state is at a maximum in free energy. When the equilibrium between reactants and products changes, the transition state moves toward the species that has increased in energy. (b) Perpendicular to the reaction pathway, the transition state is at a minimum in free energy. When the equilibrium between the phosphorane and metaphosphate species changes, the transition state moves toward the species that has decreased in energy. (c) A two-dimensional reaction coordinate diagram for symmetric phosphoryl-transfer reactions with alkyl nucleophiles and leaving groups, with approximate predicted changes in transition-state structure as ester substituents are added to the phosphoryl group and the energy of the phosphorane corner decreases. (d) Similar approximate predictions for symmetric reactions with p-nitrophenolate nucleophiles and leaving groups. Whereas monoester reactions show little variability in transition state, triesters show considerable variability (as discussed further in Supplemental Section C).