Abstract
Plants in their natural environments are constantly subjected to biotic stress. In addition to possessing physical barriers and anti-nutritive toxins, plants can be primed to respond more efficiently against future attack via faster and stronger gene activation. Here we discuss recent findings showing that plants can pass signatures of attack to the next generation, thus rendering the progeny more resistant against insect and pathogen attack. A combination of phytohormone signaling, small RNA-mediated gene silencing and DNA methylation are involved in transgenerational induced resistance. Epiallelic variation against biotic threats should be under positive selection in populations of plants where the environment is predictable over time. Similarly, in very genetically homogenous populations, such as during range expansion, epigenome reorganization is a likely mechanism for faster plant adaptation to novel biotic attack. Further research is needed to understand the relative role of the genome vs. the epigenome for the evolution of increased plant resistance.
Keywords: plant biotic stresses, adaptation, epigenetic, plant resistance, range expansion, small RNAs
Plant Immunity Against Biotic Stress
Adaptation to environmental stress is essential for survival and propagation in diverse ecological landscapes. Plants have evolved a large variety of adaptations to counteract herbivore or pathogen pressure, including constitutive physical and chemical barriers,1 induction of toxic or anti-nutritive proteins or secondary metabolites after attack2 and priming for faster and stronger response against future attacks.3 Priming for enhanced responses to subsequent biotic attack has been argued to be relatively cost-free4 and is of particular value if plants can predict the future environments to which they, or even their progeny, will be exposed.5
Epigenetics of Transgenerational Increased Plant Resistance Against Biotic Stress
Due to the likely role in adaptation, there is an increasing interest in studying the transgenerational inheritance of environmentally induced changes that can confer increased biotic stress tolerance. Such inherited resistance requires epigenetic mechanisms that alter transcriptional activities and can be transmitted through meiosis to subsequent generations.6 Three recent studies7-9 indicate that epigenetic mechanisms, such as DNA methylation, chromatin remodeling and siRNA synthesis play a central role in regulating transgenerational plant immune responses. Biotic stress from insects and pathogens, as well as plant defense signaling molecules such as jasmonic acid or salicylic acid, can elicit the production of small RNAs (sRNAs)10 and methylation changes that lead to epiallelic variation in the Arabidopsis thaliana (Arabidopsis) genome.7
Variation in Epigenomic Variability
During the past decade, several studies have reported environmentally triggered acquisition of new, heritable plant traits.11-14 In some cases, phenotypic changes could be observed over several generations.7,9,11,12,15,16 Analysis of natural accessions and mutant plants shows that there is variation in the acquisition of heritable traits after both biotic and abiotic treatments.5,17,18 For example, exposure to physical and chemical stresses in Arabidopsis significantly increases somatic homologous recombination numbers, which is related to the expression level of genes involved in homologous recombination and repair.19 However, in this same study, two subsequent non-treated generations showed low and stochastic increases in somatic homologous recombination that did not correlate with the degree of stimulation in the parental plants.19 Such results indicate within-species variability in the epigenetic inheritance of resistance traits in plants.16 Also, at least in the model plant Arabidopsis, transgenerational effects are not a general stress response and may require special conditions that cannot be regulated precisely, even in a very controlled laboratory setting.9
Merging Epigenetic Variation in Plants with Ecological Research
For plants under biotic attack, epigenetic transgenerational modifications undoubtedly have the potential to increase the ability of progeny to adapt to environmental challenges. This, however, can only be true if environments with threatening biotic factors change less rapidly than the generation time of the plant that is affected. For instance, in the case of plants with no dormancy period and seeds that germinate next to the parent plant, the maternal environment is a good predictor of the progeny environment. For seeds that overwinter and germinate in the following spring, there may still be predictive value if high herbivore pressure in one year is likely to be followed by large numbers of herbivores in the following year. We thus hypothesize that, across plant species, those with low dispersal will be selected for increased transgenerational epiallele utilization in herbivore resistance compared with highly dispersive species. Additionally, given differential predictive value of the parental environment, it is likely that species expanding into new environments with high and constant threats should be subjected to selection for enhanced stress tolerance at the epigenetic level. Such selection could occur rapidly and should also be reversible if there are changes in the environment. Thus, given the strong plastic responses that a plant can have via heritable epigenetic changes, it is very likely that the epigenome is under natural selection (Fig. 1). However, the extent to which the genome, methylome and transcriptome vary across the natural growth range of different plant species remains to be tested. Investigation of such natural variation in plant epigenetic stress responses will likely be an area of future research. Currently available high-throughput sequencing tools make it possible to dissect the relative importance of genome mutations and rearrangements vs. epigenetic changes. Such studies should include species that have colonized diverse environments, in which populations experience strong ecological gradients and variation in the biotic and abiotic stress conditions. Subsequently, understanding the role of epigenetic processes driving the evolution of plant stress tolerance, and then linking these to plant defensive traits, will offer interesting opportunities for plant breeders. It may be possible to select for epigenetic changes or long-term responses to stress treatments that will provide growers with more resistant crop genotypes that require less energetic and environmentally costly inputs.20
Figure 1.
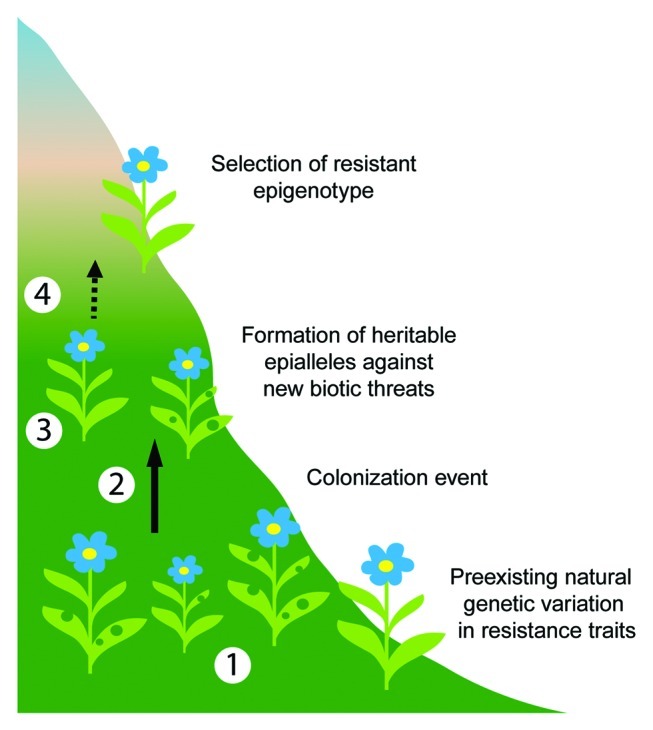
Evolution of plant resistance through alteration of epigenetic regulation. (1) A large natural plant population displaying phenotypic variation in resistance traits due to preexisting natural genetic variation (represented here by variation in the number of leaf holes). (2) Large populations tend to expand into new habitats. In this example, it is expected that alpine plants will colonize higher altitudes due to global climate change. (3) After colonization, new populations with limited and more homogenous genetic diversity will form. When faced with new biotic threats, plants in the novel population will react with a destabilization of epigenetic regulation. Alteration of epigenetic regulation can lead to the formation of heritable epialleles. (4) Epiallelic-driven variation in gene expression generates phenotypic diversity for natural selection to favor more resistant plants. Ideas inspired by Mirouze and Paszkowski.17
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/19525
References
- 1.Schoonhoven LM, van Loon JJA, Dicke M. Insect-plant biology. Oxford: Oxford University Press, 2005. [Google Scholar]
- 2.Karban R, Baldwin IT. Induced Responses to Herbivory. Chicago: The University of Chicago Press, 1997. [Google Scholar]
- 3.van Loon LC, Bakker PA, Pieterse CMJ. Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol. 1998;36:453–83. doi: 10.1146/annurev.phyto.36.1.453. [DOI] [PubMed] [Google Scholar]
- 4.van Hulten M, Pelser M, van Loon LC, Pieterse CMJ, Ton J. Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci U S A. 2006;103:5602–7. doi: 10.1073/pnas.0510213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agrawal AA. Transgenerational consequences of plant responses to herbivory: an adaptive maternal effect? Am Nat. 2001;157:555–69. doi: 10.1086/319932. [DOI] [PubMed] [Google Scholar]
- 6.Chinnusamy V, Zhu JK. Epigenetic regulation of stress responses in plants. Curr Opin Plant Biol. 2009;12:133–9. doi: 10.1016/j.pbi.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luna E, Bruce TJA, Roberts MR, Flors V, Ton J. Next Generation Systemic Acquired Resistance. Plant Physiol. 2012;158:844–53. doi: 10.1104/pp.111.187468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slaughter A, Daniel X, Flors V, Luna E, Hohn B, Mauch-Mani B. Descendants of primed Arabidopsis plants exhibit resistance to biotic stress. Plant Physiol. 2012;158:835–43. doi: 10.1104/pp.111.191593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasmann S, De Vos M, Casteel CL, Tian D, Halitschke R, Sun JY, et al. Herbivory in the previous generation primes plants for enhanced insect resistance. Plant Physiol. 2012;158:854–63. doi: 10.1104/pp.111.187831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosher RA, Melnyk CW. siRNAs and DNA methylation: seedy epigenetics. Trends Plant Sci. 2010;15:204–10. doi: 10.1016/j.tplants.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Molinier J, Ries G, Zipfel C, Hohn B. Transgeneration memory of stress in plants. Nature. 2006;442:1046–9. doi: 10.1038/nature05022. [DOI] [PubMed] [Google Scholar]
- 12.Verhoeven KJF, Jansen JJ, van Dijk PJ, Biere A. Stress-induced DNA methylation changes and their heritability in asexual dandelions. New Phytol. 2010;185:1108–18. doi: 10.1111/j.1469-8137.2009.03121.x. [DOI] [PubMed] [Google Scholar]
- 13.Whittle CA, Otto SP, Johnston MO, Krochko JE. Adaptive epigenetic memory of ancestral temperature regime in Arabidopsis thaliana. Botany. 2009;87:650–7. doi: 10.1139/B09-030. [DOI] [Google Scholar]
- 14.Boyko A, Blevins T, Yao Y, Golubov A, Bilichak A, Ilnytskyy Y, et al. Transgenerational adaptation of Arabidopsis to stress requires DNA methylation and the function of Dicer-like proteins. PLoS One. 2010;5:e9514. doi: 10.1371/journal.pone.0009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whittle CA, Otto SP, Johnston MO, Krochko JE. Adaptive epigenetic memory of ancestral temperature regime in Arabidopsis thaliana. Botany. 2009;87:650–7. doi: 10.1139/B09-030. [DOI] [Google Scholar]
- 16.Kathiria P, Sidler C, Golubov A, Kalischuk M, Kawchuk LM, Kovalchuk I. Tobacco mosaic virus infection results in an increase in recombination frequency and resistance to viral, bacterial and fungal pathogens in the progeny of infected tobacco plants. Plant Physiology 2010:pp.110.157263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirouze M, Paszkowski J. Epigenetic contribution to stress adaptation in plants. Curr Opin Plant Biol. 2011;14:267–74. doi: 10.1016/j.pbi.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Grativol C, Hemerly AS, Ferreira PCG, Berlinger M, Hirt H, Luschnig C, et al. Genetic and epigenetic regulation of stress responses in natural plant populations. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms. 2012;1819:176–85. doi: 10.1016/j.bbagrm.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Pecinka A, Rosa M, Schikora A, Berlinger M, Hirt H, Luschnig C, et al. Transgenerational stress memory is not a general response in Arabidopsis. PLoS One. 2009;4:e5202. doi: 10.1371/journal.pone.0005202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauben M, Haesendonckx B, Standaert E, Van Der Kelen K, Azmi A, Akpo H, et al. Energy use efficiency is characterized by an epigenetic component that can be directed through artificial selection to increase yield. Proc Natl Acad Sci U S A. 2009;106:20109–14. doi: 10.1073/pnas.0908755106. [DOI] [PMC free article] [PubMed] [Google Scholar]


