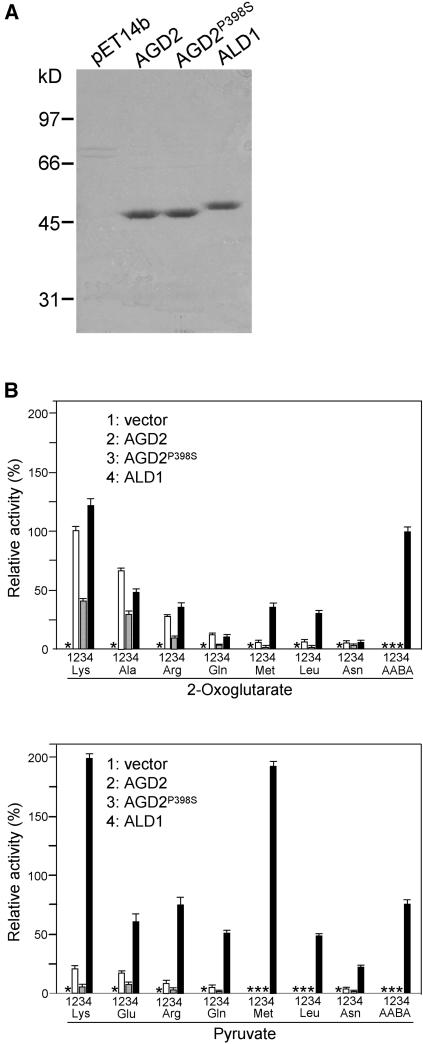
Figure 6.
Purification and Relative Activities of AGD2, AGD2P398S, and ALD1.
(A) Electrophoretic patterns of purified recombinant proteins through column elution using Ni2+-NTA.
(B) Relative aminotransferase activities of AGD2, AGD2P398S, and ALD1. Substrates were at a 50-mM concentration. Aminotransferase activity was determined by measuring concentration of the reaction product Glu (using 2-oxoglutarate as the cosubstrate) or Ala (using pyruvate as the cosubstrate). The activity of AGD2 with Lys and 2-oxoglutarate was arbitrarily set at 100%. Asterisks indicate no detectable activities. No detectable activity was seen with all of the other standard amino acids tested. Bars indicate standard deviations (n = 3).