Figure 7. Effect of BRCA2 mutation on Alix and Tsg101 localization and cytokinesis.
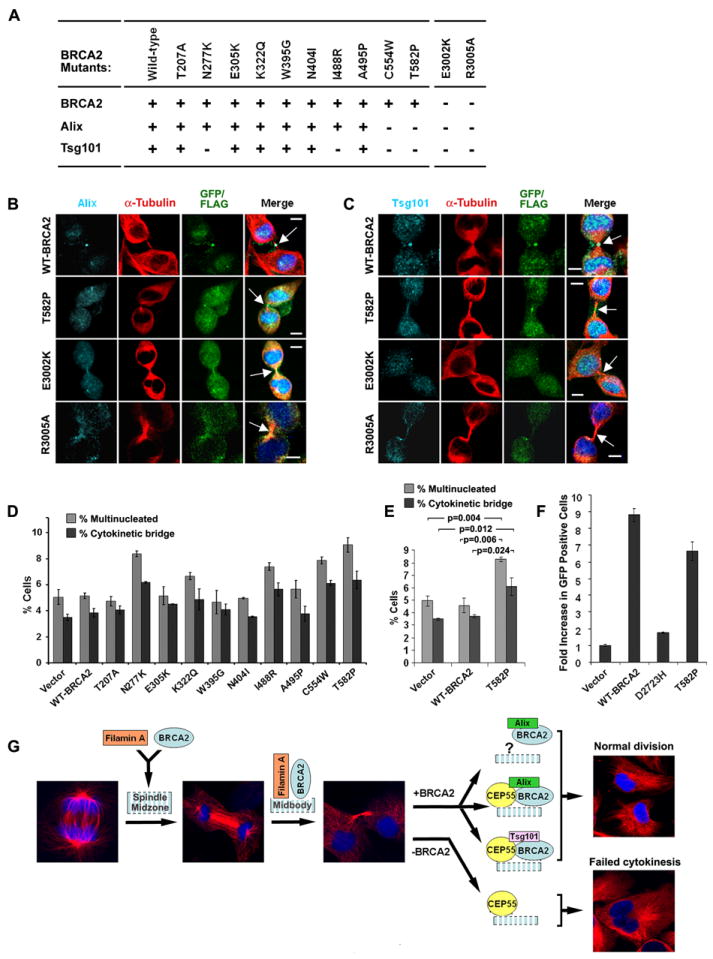
(A) Summary of midbody localization of BRCA2, Alix and Tsg101 in response to BRCA2 missense mutants. “+” normal and “−” reduced localization. (B-C) Disruption of Alix (B) and Tsg101 (C) midbody localization by BRCA2 mutations shown by immunofluorescence (IF) staining for Alix and Tsg101 in 293T cells expressing FLAG or GFP-tagged WT and mutant BRCA2. Arrows indicate midbodies. Bars (B, C), 5μm. (D-E) Bar graph of percent 293T cells with multiple nuclei and unresolved cytokinetic bridges 72 hr after transfection with the indicated BRCA2 constructs; p-values (E) indicate the statistical significance of the differences. (F) Rescue of homology directed repair activity of BRCA2 mutants in brca2 deficient DR-GFP V-C8 cells. GFP positive cells were identified by FACS analysis following co-transfection with I-Sce1 and WT or mutant BRCA2 vectors. Error bars (in D-F) show standard error of the mean. (G) Model of BRCA2 activity at the midbody. Stages of progression from anaphase through completion of cytokinesis are shown by α-tubulin and DAPI staining. BRCA2 is recruited to the midbody by Filamin A during furrow ingression and cytokinetic bridge formation, and interacts with CEP55 to assist the formation of CEP55-Alix and CEP55-Tsg101 complexes. Impairment of BRCA2 function reduces the availability of these complexes at the midbody leading to abnormal or failed cytokinesis. (See also Figure S7).
