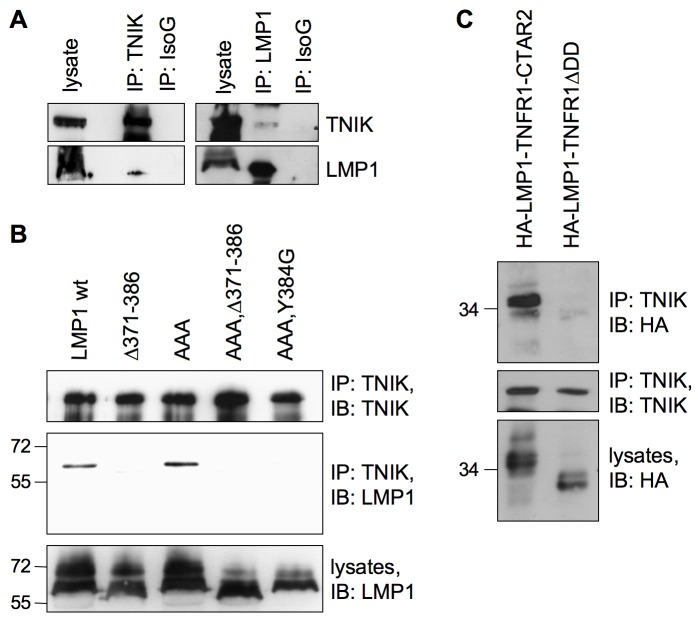
Figure 2. TNIK binds to the LMP1 signaling complex.
(A) Endogenous TNIK interacts with LMP1 in lymphoblastoid cells. TNIK was immunoprecipitated from LCL 721 cell lysates with the anti-TNIK antibody (left panel). Co-precipitated wildtype LMP1 was detected by the anti-LMP1 (1G6-3) antibody. An unrelated mouse isotype IgG (IsoG) served as a negative control for immunoprecipitation. Vice versa, endogenous LMP1 was immunoprecipitated using the anti-LMP1 (1G6-3) antibody (right panel). Co-precipitated TNIK was detected using the anti-TNIK antibody. Rat isotype IgG served as a negative control. IB, immunoblot; IP, immunoprecipitation. Shown is one representative of four independent experiments (n = 4). (B) The CTAR2 domain of LMP1 is essential for TNIK recruitment. HEK293 cells were transfected in 15 cm culture dishes with 20 µg of the indicated LMP1 constructs. The Δ371-386 deletion inactivates the CTAR2 domain. CTAR2 with Y384G mutation has no signaling capacity towards NF-κB and JNK. AAA harbors a mutated TRAF interaction motif within CTAR1. Endogenous TNIK was immunoprecipitated from cell lysates. LMP1 co-precipitation was analyzed using the anti-LMP1 (CS1-4) antibody. The anti-TNIK antibody was used to verify equal TNIK precipitation in all samples. Comparable expression of LMP1 constructs was confirmed in cell lysates. n = 3. (C) The 16 C-terminal amino acids of CTAR2 are sufficient for interaction with TNIK. HEK293 cells were transfected with pCMV-HA-LMP1-TNFR1ΔDD, a chimera of the LMP1 transmembrane domain and the signaling domain of TNFR1 lacking its death domain (DD), or pCMV-HA-LMP1-TNFR1-CTAR2 carrying amino acids 371–386 of LMP1 instead of DD. Endogenous TNIK was immunoprecipitated from cell lysates and co-precipitation of the HA-tagged chimeras was analyzed by immunoblotting with the anti-HA (3F10) antibody. The anti-TNIK antibody was used to confirm comparable TNIK precipitation. n = 3.