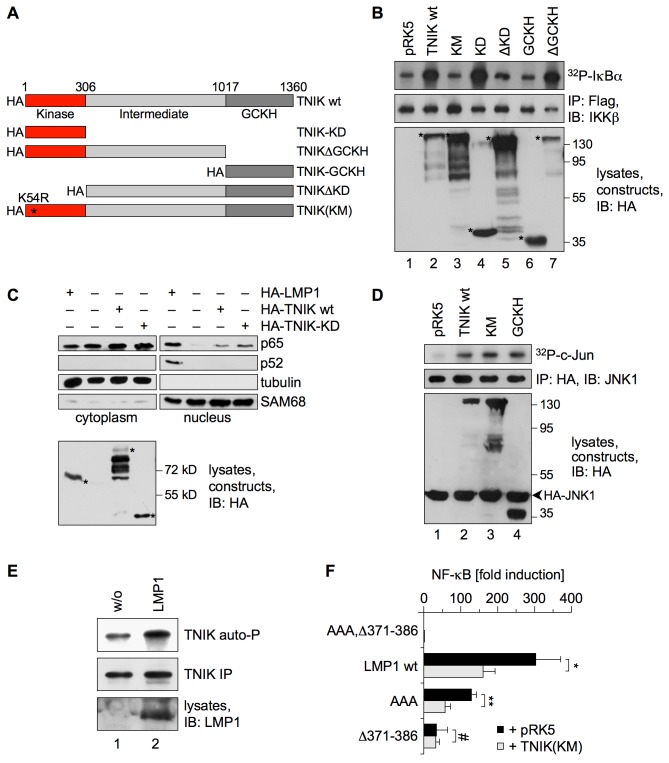
Figure 6. The JNK and canonical NF-κB pathways bifurcate at the level of TNIK.
(A) Schematic diagram of TNIK mutants cloned with an N-terminal HA-tag. Amino acid positions are given in numbers. The asterisk marks the position of the K54R mutation that inactivates the kinase. GCKH, germinal center kinase homology domain; KD, kinase domain; KM, kinase mutant. (B) The intact TNIK kinase domain is required for IKKβ activation. HEK293 cells were transfected in 6-well plates with 2 µg of the indicated TNIK constructs or pRK5 empty vector and 1 µg of Flag-IKKβ. Flag-IKKβ activity was monitored in immunocomplex kinase assays. Immunoprecipitated Flag-IKKβ and expressed TNIK-constructs were detected using the anti-IKKβ and anti-HA (3F10) antibodies, respectively. Asterisks indicate the TNIK constructs. Apparent molecular masses are given in kDa. Quantification of three independent experiments: lane 1, 1.0±0.0; lane 2, 4.73±1.0; lane 3, 0.93±0.46; lane 4, 11.0±5.86; lane 5, 1.3±0.56; lane 6, 1.4±0.62; lane 7, 5.13±1.72. (C) The TNIK kinase domain induces the nuclear shift of canonical p65 NF-κB but not of non-canonical p52 NF-κB. HEK293 cells were transfected in 10 cm dishes with 4 µg each of the indicated constructs. LMP1 served as positive control. Cytoplasmic and nuclear fractions were prepared and analyzed by immunoblotting using the indicated antibodies. Expression of the constructs was verified in total lysates by the anti-HA (3F10) antibody. n = 4. (D) The GCKH domain of TNIK is sufficient for JNK activation and TNIK kinase activity is dispensable. HEK293 cells were transfected with 0.5 µg of the indicated HA-tagged TNIK constructs or pRK5 empty vector and 1 µg of HA-JNK1. Subsequently, immunocomplex kinase assays were performed. Immunoprecipitated HA-JNK1 and the expressed TNIK-constructs were detected using the anti-JNK1 and anti-HA (3F10) antibodies. Quantification of three independent experiments: lane 1, 1.0±0.0; lane 2, 3.2±1.66; lane 3, 4.57±1.35; lane 4, 6.0±0.1. (E) LMP1 enhances autophosphorylation of TNIK. HEK293 cells were transfected in 6-well plates with 1 µg of HA-TNIK vector together with or without 1 µg of pSV-LMP1, as indicated. Subsequently, HA-TNIK was immunoprecipitated from cell lysates using the anti-HA (12CA5) antibody and TNIK autophosphorylation reaction was performed with the precipitated TNIK in the presence of radioactive ATP. Equal TNIK precipitation and LMP1 expression was confirmed on immunoblots. Quantification of six independent experiments: lane 1, 1.0±0.0; lane 2, 4.28±2.76; two-tailed Student's t test, p = 0.015. (F) Kinase-negative TNIK(KM) exerts a dominant-negative effect specifically on the NF-κB pathway induced by CTAR2. HEK293 cells were transfected in 6-well plates with 1 µg of the indicated LMP1 constructs, 2 µg of kinase-negative HA-TNIK(KM) or empty vector, and 5 ng of the NF-κB reporter 3xκBLuc and Renilla control reporter. Luciferase activities were corrected for transfection efficiencies. Given data are mean values of three independent experiments ± standard deviations; two-tailed Student's t test. *p = 0.03, **p = 0.003, # p = 0.88.