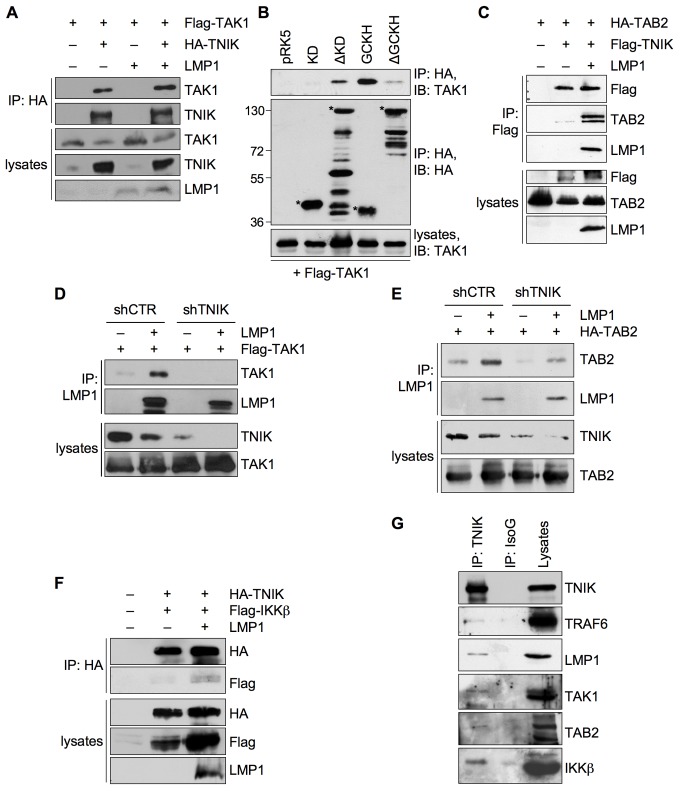
Figure 8. TNIK forms a dynamic signaling complex with TAK1, TAB2, and IKKβ upon LMP1 activation.
(A) TAK1 interacts with TNIK independent of LMP1. HEK293 cells were transfected in 15 cm culture dishes with 5 µg of HA-TNIK and 2 µg of Flag-TAK1 vectors, both in the presence and absence of 5 µg pSV-LMP1, as indicated. HA-TNIK was immunoprecipitated with the anti-HA (12CA5) antibody. Immunoprecipitations were analyzed using anti-TNIK and anti-TAK1 antibodies. Cell lysates were stained with anti-TNIK, anti-TAK1, and anti-LMP1 (CS1-4) antibodies. n = 3. (B) TAK1 binds to the GCKH and intermediate domains of TNIK. HEK293 cells were transfected in 10 cm cell culture dishes with 2 µg of the indicated HA-TNIK constructs and 1 µg of Flag-TAK1 vector. HA-TNIK constructs were immunoprecipitated with the anti-HA (12CA5) antibody. Immunoprecipitations and lysates were analyzed with anti-HA (3F10) and anti-TAK1 antibodies, as indicated. Asterisks indicate TNIK proteins. Apparent molecular masses are given in kDa. n = 4. (C) LMP1 induces the interaction between TNIK and TAB2. HEK293 cells were transiently transfected in 15 cm culture dishes with 5 µg of HA-TAB2 and Flag-TNIK vectors in the presence or absence of 5 µg pSV-LMP1 as indicated. Flag-TNIK was immunoprecipitated using the anti-Flag (6F7) antibody. The following antibodies were used for immunoblotting: anti-Flag (6F7), anti-TAB2, anti-LMP1 (1G6-3). n = 3. (D) TNIK is essential for TAK1 interaction with LMP1. HEK293 cells were transfected in 15 cm culture dishes with 5 µg Flag-TAK1, 3 µg LMP1, and 7 µg each of shTNIK or shCTR vectors, as indicated. Cells were lysed 48 h post-transfection, LMP1 was immunoprecipitated using the anti-LMP1 (1G6-3) antibody, and co-precipitation of Flag-TAK1 was analyzed by immunoblotting for TAK1. TNIK knockdown and Flag-TAK1 expression was verified in total cell lysates. n = 3. (E) TNIK mediates the interaction between TAB2 and LMP1. HEK293 cells were transfected with the indicated vectors as described in (D). LMP1 was precipitated from cell lysates using the anti-LMP1(1G6-3) antibody. Immunoprecipitations and lysates were analyzed with the indicated antibodies. n = 2. (F) LMP1 induces the recruitment of IKKβ to the TNIK complex. HEK293 cells were transfected in 10 cm culture dishes with 2 µg HA-TNIK, 2 µg Flag-IKKβ, and 3 µg pSV-LMP1 as indicated. HA-TNIK was immunoprecipitated using the anti-HA (12CA5) antibody and immunocomplexes were analyzed by immunoblotting using anti-HA (12CA5) and anti-Flag (M2) antibodies. Expression of HA-TNIK, Flag-IKKβ, and LMP1 was detected in cell lysates with the anti-HA (12CA5), anti-Flag (M2), and anti-LMP1 (1G6-3) antibodies. n = 3. (G) TNIK signaling complex containing LMP1, TRAF6, TAK1, TAB2, and IKKβ in EBV-transformed lymphoblastoid cells. Endogenous TNIK was immunoprecipitated from LCL 721 cells with the anti-TNIK antibody. An unrelated mouse isotype IgG was used for control precipitation (IsoG). As indicated, immunoprecipitations and lysates were analyzed by immunoblotting using the anti-TNIK, anti-TRAF6 (H-274), anti-LMP1 (1G6-3), anti-TAK1, anti-TAB2, and anti-IKKβ antibodies. n = 2.