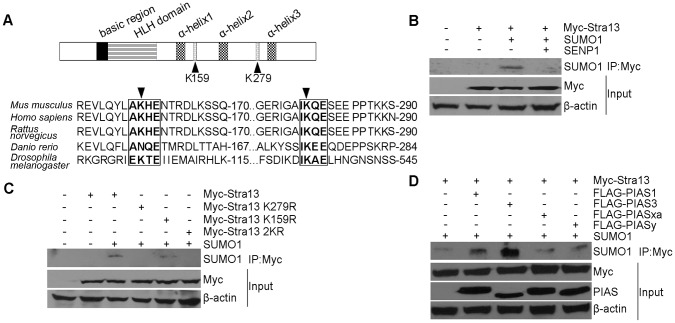
Figure 1. Stra13 is sumoylated.
(A) Schematic representation of the Stra13 domain structure (upper panel). The basic and HLH domains are shown along with three α-helices in the C-terminal repression domain. Potential sumoylation acceptor lysines at 159 and 279 (K159 and K279) are indicated. Numbers indicate amino acid residues in the mouse Stra13 cDNA. Alignment of Stra13 cDNA from various species revealed a highly conserved SUMO consensus motif IKQE, and a somewhat less conserved motif AKHE that are highlighted. K159 and K279 are indicated by arrowheads (lower panel). (B) Cells were co-transfected with Myc-Stra13, SUMO1 and SENP1 as indicated. Lysates were immunoprecipitated with Myc-agarose beads followed by immunoblotting with anti-SUMO1 antibody. Input shows expression of Stra13 using anti-Myc antibody. β-actin served as a loading control. (C) Cells were co-transfected with Myc-Stra13, or point mutants (Stra13 K279R, Stra13 K159R, Stra13 2KR) together with SUMO1. Cell lysates were immunoprecipitated with Myc-agarose beads and the immunoprecipitates were subjected to western blotting with anti-SUMO1 antibody. (D) Myc-Stra13 and SUMO1 were expressed along with Flag-PIAS1, PIAS3, PIASxα, or PIASy as indicated. Lysates were immunoprecipitated with Myc- agarose beads followed by western blotting with anti-SUMO1 antibody. Lysates (input) were probed for Stra13 and PIAS.