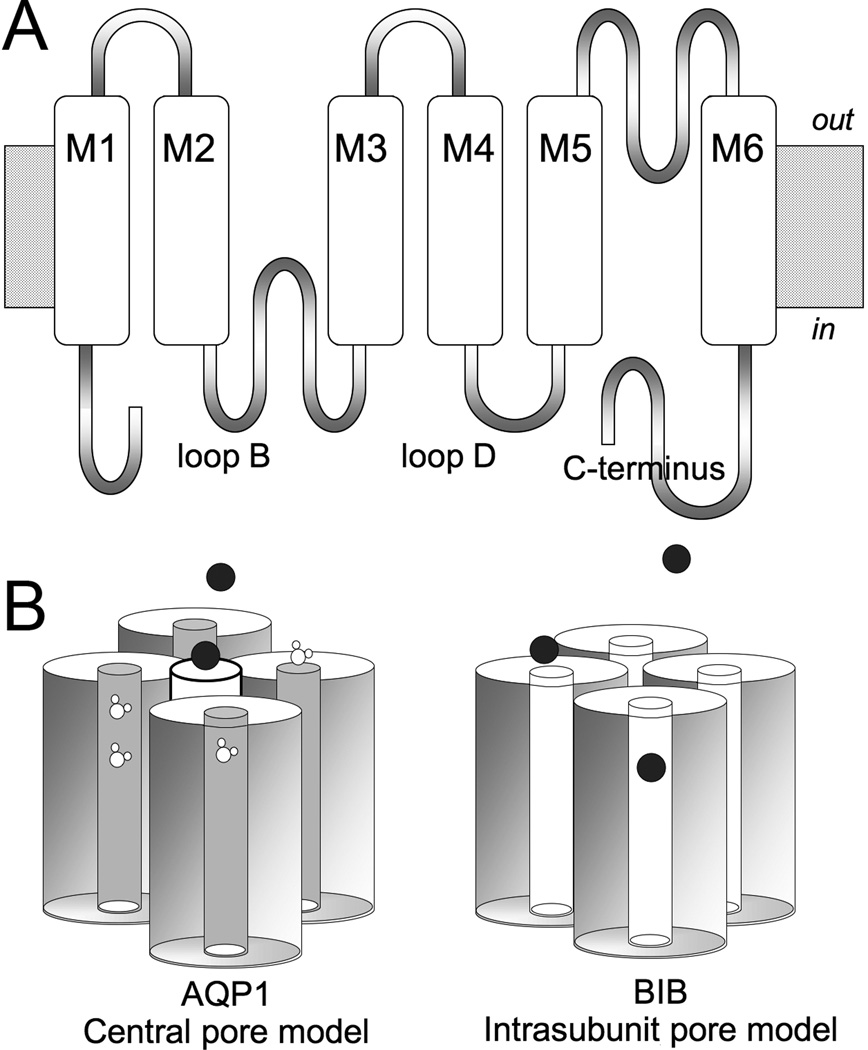
Figure 1.
Diagram illustrating the Aquaporin channel structural organisation and candidate permeation pathways.
(A) Transmembrane topology of an aquaporin subunit, with six transmembrane domains (M1-6), intracellular amino and carboxyl terminal domains, and two folded loops (B and E) that meet within the transmembrane region of the channel to create the intrasubunit pore of each subunit.
(B) Two models for ion permeation. Ions can pass through the central pore in the middle of the tetramer of subunits (as has been shown for Aquaporin-1, AQP1), or might through the individual intrasubunit pore pathways of aquaporin channels (as has been proposed for Drosophila Big Brain, BIB, and others). Water flux in AQP1 is mediated primary by the intrasubunit pores. BIB has no appreciable osmotic water permeability.