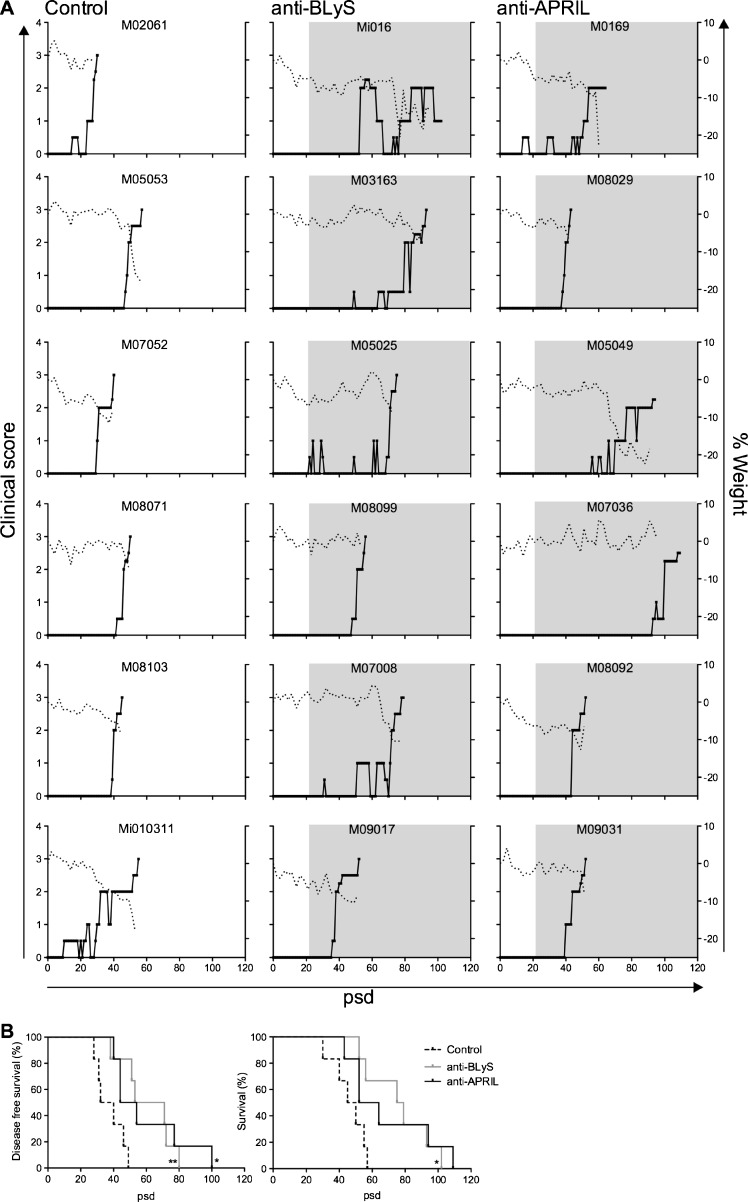
Fig. 3.
Delay of EAE course by anti-BLyS and anti-APRIL treatment. a. Clinical scores are depicted of controls (left panel) and the two antibody treated groups, with anti-BlyS (middle panel) or anti-APRIL (right panel). The solid lines represent clinical scores (left y-axes) and the dotted line body weight loss relative to the immunization day, defined as post sensitization day (psd) 0. Grey shaded boxes indicate the treatment period. All animals in the experiment developed clinically evident EAE (clinical score 2.0) and most of them were sacrificed with an EAE score of 3.0. However, monkey Mi016, M0169 and M05049 had to be sacrificed at an earlier time point due to the serious body weight loss. b. Survival curves depict the disease free survival time (time interval to development of EAE score 2.0; left panel) and overall survival (time interval to clinical end point; right panel). Disease free survival times were significantly prolonged in the anti-BLyS and anti-APRIL treated monkeys. The total survival was significantly prolonged by the anti-BLyS treatment, but the delay in anti-APRIL treated monkeys was not significant (p = 0,0646). *p < 0.05; **p < 0.001 Log-rank test, treated group vs. control group