Abstract
Having failed to respond to an adequate antidepressant treatment course predicts poorer treatment outcomes in patients with major depression. However, little is known about the impact of prior treatment on the outcome of major depression with psychotic features (MDpsy). We examined the effect of prior treatment history on the outcome of pharmacotherapy of MDpsy in patients who participated in the STOPD-PD study, a randomized, double-blind, clinical trial comparing a combination of olanzapine plus sertraline vs. olanzapine plus placebo. The strength of treatment courses received prior to randomization was classified using a validated method. A hierarchy of outcomes was hypothesized based on treatments received prior to randomization and randomized treatment. A high remission rate was observed in subjects with a history of no prior treatment or inadequate treatment who were treated with a combination of olanzapine and sertraline. A low remission rate was observed in subjects who had previously failed to respond to an antidepressant alone and who were treated with olanzapine monotherapy. A low remission rate was also observed in subjects who had previously failed to respond to a combination of an antipsychotic and an antidepressant. Similar to patients with major depression, these results emphasize the impact of prior pharmacotherapy on treatment outcomes in patients with MDpsy.
Keywords: Psychotic Depression, Pharmacotherapy, Treatment Resistance
INTRODUCTION
Major Depressive Disorder (MDD) is a highly prevalent mental illnesses in North America (Kessler et al., 2003; Patten et al., 2006). Major depression with psychotic features (MDpsy) is a severe form of major depressive disorder (MDD) that carries significant morbidity and a poor prognosis (Coryell et al., 1996; Rothschild, 2003). Thus, clinicians require indicators of treatment response prior to initiating a management plan. Prior treatment has been shown to predict remission and response of MDD when patients receive electroconvulsive therapy (ECT) (Prudic et al., 1996) or psychopharmacological treatment (Dombrovski et al., 2005; Rasmussen et al., 2007; Tew et al., 2006). In particular, patients who fail to respond to a previous adequate course of pharmacotherapy are significantly less likely to respond to treatment than treatment naïve patients or than those who have been exposed to an inadequate course (Amsterdam et al., 2009; Hennings et al., 2009; Tew et al., 2006). Thus, accurate assessment of treatment resistance and differentiating it from inadequate treatment has clinical implications for the treatment of MDD. However, little is known about the impact of prior treatment on the outcome of MDpsy.
We conducted this analysis to examine the impact of prior treatment history on the outcome of pharmacotherapy of MDpsy in patients who participated in a randomized clinical trial comparing under double-blind conditions a combination of olanzapine plus sertraline vs. olanzapine plus placebo(Meyers et al., 2009), referred to in this manuscript as combination and monotherapy. We used a validated and reliable method to classify the strength of treatment courses received prior to randomization(Andreescu et al., 2007; Oquendo et al., 2003; Oquendo, Malone, Ellis, Sackeim, & Mann, 1999). We hypothesized a hierarchy of outcomes based on the adequacy of treatments received during the index episode and treatment assignment during the study. Specifically, we hypothesized that patients who had not received any prior treatment and who were randomized to combination therapy would have a high rate of remission; those who had been treated with an adequate combination of an antidepressant and an antipsychotic and who were randomized to monotherapy would have a low rate of remission; and those who had failed to respond to an antidepressant or an antipsychotic but not both, would have an intermediate rate of remission.
METHODS
Subjects
As described previously (Meyers et al., 2009), patients 18 years of age or older admitted to the inpatient or ambulatory services of four academic sites between December 2002 and June 2007 were eligible for participation in the study. The Institutional Review Boards of the four institutions and a Data Safety Monitoring Board at the National Institute of Mental Health approved study consent forms and monitored the study's progress. Informed consent was obtained from all subjects, either directly or through locally approved substitute decision makers.
Strategies to identify eligible patients included review of new admissions, advertisements, and direct referrals by community psychiatrists. Subjects were assessed with the Structured Interview for Clinical Diagnosis (SCID) (First & et al., 2002) to assure that DSM-IV-TR criteria for unipolar MDpsy were met. Other inclusion criteria included: the presence of at least one delusional belief defined as a fixed idea that was held contrary to the laws of logic; a score of ≥3 on the delusion severity rating item of the Schedule of Affective Disorders and Schizophrenia (SADS) (Endicott & Spitzer, 1978) (meaning that subjects had no more than a transient ability to consider the implausibility of their irrational belief); a score of ≥2 on one of the conviction items of the Delusional Assessment Scale (DAS) (Meyers et al., 2006); and a score ≥21 on the 17 item Hamilton Depression Scale (Ham-D) (Hamilton, 1960), which was administered using the GRID-Ham-D method (Williams et al., 2008). Exclusion criteria included: immediate indication for ECT because of refusal to eat or drink or imminent risk for suicide (however, patients with current suicidal ideation without immediate intent and those who had made a suicide attempt during the current episode were allowed to begin the study on an inpatient basis); a dementia or a history of impaired cognition prior to the current depressive episode; meeting criteria for another Axis I psychotic or mood disorder, current body dysmorphic disorder or obsessive-compulsive disorder, or substance abuse during the preceding three months; the presence of an unstable medical condition that might interfere with completion of the twelve-week trial; a neurological disease, such as Parkinson's disease, that might affect neuromuscular functioning; ongoing need for medications known to cause depression or psychosis; having received olanzapine 15 mg/day or more for a minimum of four weeks during the current episode; and benefiting from one's current psychotropic medications. Patients with known hyperlipedemia or diabetes mellitus, including insulin-dependent diabetes, were allowed to enrol if their metabolic conditions were stable. Screening also involved baseline laboratory assessments, including TSH, folate and B12 levels, an electrocardiogram, and a toxicology screen to detect undisclosed illicit drug use.
Intervention
Eligible subjects were randomized using computer-generated lists with investigators and raters blind to treatment assignments. Randomization was stratified by site and age ≥60 with a block size of four. Subjects taking antidepressant or antipsychotic medications at entry had these tapered prior to randomization but a wash out period was not enforced because of the severity of illness anticipated in study participants. Subjects began 2.5–5mg/day of olanzapine and 25–50 mg/day of sertraline or matching placebo, with dose increases permitted every three days as tolerated. Olanzapine was administered openly and sertraline or placebo under double-blind conditions. An attempt was made to reach minimum doses of 10 mg/day of olanzapine and 100 mg/day of sertraline or placebo before the end of week one. Doses were increased to 15 mg/day of olanzapine and 150 mg/day of sertraline or placebo during week two, with further increases allowed to a maximum of 20 mg/day of olanzapine or 200 mg/day of sertraline, as tolerated, beginning in week three. Slower titration or temporary dose reductions of one or both medications was allowed if side effects were suspected; however, subsequent attempts to achieve minimum daily target doses of 15 mg/day of olanzapine and 150 mg/day of sertraline or placebo were required. Adjunctive lorazepam up to 4 mg/day was allowed to control anxiety or agitation and benztropine up to 2 mg/day to control extrapyramidal symptoms. No other psychotropics were allowed.
Clinical Assessments
Baseline assessments were completed within seven days of obtaining consent. Follow-up research assessments were conducted weekly for the first six weeks and then every other week until week twelve or termination. Research assessments included overall symptom severity using the Clinical Global Illness Scale for severity (CGI-S) (Guy, 1976), Ham-D, assessments for delusional ideation using the DAS and the SADS delusional item. At baseline, the Cumulative Illness Burden Scale (Miller et al., 1992) was used to assess general medical burden and the Mini-Mental-State-Examination (MMSE) (Folstein, Folstein, & McHugh, 1975) was used to assess global cognitive functioning. Raters were trained to achieve adequate reliability prior to conducting study assessments and inter-rater reliability reassessed annually thereafter.
Assessment of Strength of Prior Antidepressant Treatment Courses
The strength of each pharmacological course received by the subjects during their current episode prior to enrolment (i.e., under usual clinical conditions) was assessed with a modified version of the Antidepressant Treatment History Form (ATHF) as described previously (Andreescu et al., 2007). Information regarding previous medications was obtained from all available sources: patients' reports, family reports, treating physicians, medical records, and pharmacy records. We used the original ATHF to rate the strength of antidepressant courses(Oquendo et al., 2003): the ATHF scores each antidepressant course based on the dose and the duration of treatment as: 1 (definitely inadequate); 2 (probably inadequate); 3 (probably adequate); 4 (definitely adequate); or 5 (definitely adequate antidepressant with lithium augmentation). Thus, a score of 1 corresponds to an antidepressant course of less than four weeks or a course of more than four weeks with a very low dose (e.g., sertraline less than 25 mg/day). A score of 2 corresponds to a course of more than four weeks with probably inadequate doses (e.g., fluoxetine or paroxetine between 10–19mg/day). A score of 3 corresponds to a course of more than four weeks of an antidepressant at an adequate (i.e., therapeutic) dose (e.g., venlafaxine 150–299 mg/day). A score of 4 corresponds to a course longer than four weeks with high doses of antidepressant (fluoxetine above 39 mg/day, paroxetine above 29 mg/day, sertraline above 149 mg/day, and venlafaxine above 299 mg/day).
Assessment of Strength of Prior Antipsychotic Treatment Courses
We modified the original ATHF to score antipsychotic courses and courses of a combination of an antidepressant and an antipsychotic (Andreescu et al., 2007). We did two related modifications: first, instead of rating antipsychotic courses as adequate or inadequate, we rated antipsychotic courses from 1 to 3 (1: probably inadequate; 2: intermediate; 3: probably adequate). Second, we defined cutoff points to differentiate, low, moderate, and high antipsychotic doses. All courses lasting less than three weeks, regardless of dose, were scored 1. Courses lasting three weeks or more were also scored as 1 if the dose was low; 2 if the dose was moderate; or 3 if the dose was high. Because the optimal dose of SGA in the treatment of MDpsy has not yet been well established, the cutoff points for antipsychotic doses were modified as described elsewhere (Andreescu et al., 2007). Doses of FGA and SGA from 200 to 400 mg/day CPZE (e.g., olanzapine 10 to 14.9 mg/day) were rated as 2 (moderate or “intermediate” rather than “inadequate” as in the original ATHF). We selected these cut-off points based on published results (Nelson, Price, & Jatlow, 1986; Spiker et al., 1985) and equivalence between FGA and SGA doses (Davis & Chen, 2004).
Assessment of Strength of Prior Course of Combination of Medications
In this analysis, we considered a combination of an antidepressant and an antipsychotic as adequate if an antidepressant rated 3 or higher was combined for three weeks or longer with an antipsychotic rated 2 or 3. This corresponds to a course with an adequate dose of antidepressant for a minimum of four weeks combined with an intermediate or high dose of antipsychotic for a minimum of three weeks.
Outcome criteria
As in our original analysis (Meyers et al., 2009), remission was defined as a Ham-D score of <10 at two consecutive assessments. This definition was chosen because the study was designed to recruit equal numbers of younger and older subjects; a HAM-D score of ≤10 has been a standard cut-off point for defining remission in geriatric antidepressant trials (Arean et al., 2010; Kupfer, 2005; Reynolds et al., 1996; Roose et al., 2004) and has been used in ECT studies that have included patients with MDpsy as well as both younger and older patients (Kellner et al., 2006; Petrides et al., 2001; Sackeim et al., 2001). Remission also required the absence of delusions, (SADS delusional item scores of 1) at the second assessment of the two-assessment remission of depression interval. Investigators were allowed to withdraw subjects for either clinically significant worsening or for insufficient clinical improvement (based on operationalized criteria) after five weeks of randomized treatment.
Data Analysis
Of 259 randomized subjects, we first excluded 24 subjects for whom the ATHF was not completed. We split the remaining 235 subjects in two treatment groups based on their randomization to combination therapy or to monotherapy. We also classified these 235 subjects based on their prior treatment in five groups: no treatment, inadequate treatment, adequate antidepressant, adequate antipsychotic, or adequate combination of an antidepressant and an antipsychotic. Subjects who potentially belonged to two groups (e.g., a subject who had received an adequate antidepressant course and then an adequate combination course), were classified in the “highest” group. Since only 7 subjects were classified in adequate antipsychotic group, we eliminated these 7 subjects and this group. Thus, the final analysis includes 228 subjects classified in eight groups (2 randomized treatment groups × 4 prior treatment groups). We calculated remission rates for each of these eight groups and tested our a priori hypothesis by comparing these eight remission rates using a two-tailed χ2 test with alpha was set at 0.05. Since we detected a group effect (i.e., remission rates differed significantly in at least two groups), we used χ2 tests to assess the effect of prior treatment on remission for a given treatment: we compared remission rates among the four groups treated with combination therapy (six comparisons) and among the four groups treated with monotherapy (six comparisons). The tests were two-tailed χ2 and to correct for multiple pairwise alpha was set at 0.01. All analyses were conducted using SAS version 9 (SAS Institute Inc., Cary, NC).
RESULTS
The demographic and clinical characteristics of the 228 subjects are described in Table 2.
Table 2.
Remission rates based on prior treatment and randomized treatment
| Randomized treatment | |||
|---|---|---|---|
| Treatment received prior to randomization1 | Olanzapine plus sertraline (combination therapy) (N = 119)2 | Olanzapine plus placebo (monotherapy) (N = 109)3 | |
| No treatment (N = 31) | 63.2 % (12/19) | 33.3 % (4/12) | 51.6% (16/31) |
| Inadequate treatment (N = 94) | 54.7 % (29/53) | 36.6 %(15/41) | 46.8% (44/94) |
| Adequate antidepressant (N = 80) | 20.0% (7/35) | 11.1 % (5/45) | 15% (12/80) |
| Adequate combination of an antidepressant and an antipsychotic (N = 23) | 25.0 % (3/12) | 45.5 % (5/11) | 34.8% (8/23) |
| Total 4 | 42.9% (51/119) | 26.6% (29/109) | 35.1% (80/228) |
Combination therapy vs. monotherapy: Wald χ2 = 4.20; d.f. = 1; p = 0.04. None of the four pairwise comparisons reached statistical significance (p = 0.08–0.30).
Comparison of the four groups randomized to combination therapy: Wald ξ2 = 14.10, d.f. = 3, p < 0.003. Of the six pairwise comparisons, the following were statistically significant after Bonferroni correction: No treatment vs. Adequate antidepressant (χ2 = 10.06; d.f. = 1; p <0.002). Inadequate treatment vs. Adequate antidepressant (χ2 = 10.51; d.f. = 1; p <0.002).
Comparison of the four groups randomized to monotherapy: Wald χ2 = 8.85, d.f. = 3, p = 0.031. Of the six pairwise comparisons, the following were statistically significant after Bonferroni correction: Inadequate treatment vs. Adequate antidepressant (χ2 = 7.80; d.f.=1; p < 0.006); Adequate antidepressant vs. Adequate combination (χ2 = 6.00 p = 0.01).
Wald χ2 = 19.58; d.f. = 3; p < 0.001
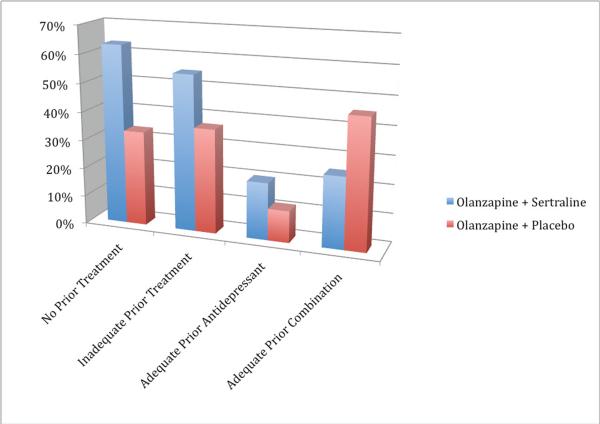
Table 2 and Figure 1 presents the remission rates based on prior and randomized treatments. Overall, there were both a treatment effect (Wald χ2 = 4.20, d.f. = 3, p = 0.04) and a group effect (Wald χ2 = 19.58, d.f. = 3, p = 0.0002). Pairwise comparisons of remission rates revealed that remission rates differed significantly between several groups (see Table 2).
Figure 1.
Remission rates based on prior treatment and randomized treatment
DISCUSSION
To our knowledge, this analysis is the first to address the impact of prior treatment exposure on pharmacotherapy of MDpsy. As predicted, a history of no prior treatment or inadequate treatment was associated with high remission rates in subjects treated with a combination of olanzapine and sertraline, while prior failure to respond to an antidepressant alone was associated with low remission rates both in subjects treated with combination therapy and in those treated with olanzapine monotherapy. However, while failure to respond to a prior adequate course of a combination of an antipsychotic and an antidepressant was associated with a low remission rate in subjects treated with combination therapy, contrary to our hypothesis, it was associated with an intermediate remission rate in subjects treated with monotherapy.
Overall, these results are congruent with what has been found in pharmacotherapy studies (Tew et al., 2006) and in some ECT studies (Sackeim et al., 2001) of patients with non-psychotic MDD: no prior treatment and inadequate prior treatment are typically associated with better outcomes, while prior failure to respond to an adequate antidepressant course is associated with worse outcomes. In other words, a higher degree of treatment resistance is associated with worse outcomes. In addition, this study extends to patients with MDpsy a finding previously reported in patients with non-psychotic MDD: inadequate treatment trials (“pseudo-treatment resistance”) is associated with a good outcome, similar to the outcome observed in treatment naïve patients (Tew et al., 2006). This emphasizes the clinical importance of distinguishing true treatment failure vs. inadequate treatment in patients who report that they have not responded to previous treatment trials.
It is important to consider that the inadequate treatment category is conflated and includes treatment that is too low in either dose or duration with either an antipsychotic or an antidepressant. The cut-offs of dose and duration for adequate antipsychotic treatment for MDpsy were derived from the literature but they have not been empirically established (Andreescu et al., 2007). Furthermore, the ATHF is a self-reported scale that relies on the accuracy of the patient's history, though raters are instructed to obtain documentation when possible. Notwithstanding these potential limitations, the current analysis suggests that patients with MDpsy who have failed to respond to prior treatment with a combination of an antipsychotic and an antidepressant have poor outcomes when they are re-challenged with a similar combination. The finding of an intermediate remission rate in patients who failed an adequate combination and who were randomized to olanzapine plus placebo is surprising and it probably reflects the small number of such subjects (n=11). However, it is possible that treatment failure with an antidepressant leads to poor outcome when subsequently treated with another antidepressant while treatment failure with an antipsychotic is not necessarily associated with a poor response to another antipsychotic. It is unlikely that one will be able to investigate this question as antipsychotic monotherapy for MDD or MDpsy is not standard. Similarly, we were not able to reliably assess the outcome of those who had failed to respond to an adequate course of antipsychotic monotherapy since there were only 7 such subjects.
In patients with schizophrenia, duration of untreated psychosis predicts poor outcomes (Keshavan et al., 2003; Schimmelmann et al., 2008). It is possible that length of untreated psychosis in MDpsy similarly confers a worse outcome. Thus, the lower remission rate seen in subjects who failed a prior adequate course of antidepressant or combination therapy could also be due to the longer duration of their current episode prior to enrolling in this trial: both groups had longer episodes than those with a history of no prior treatment or inadequate treatment (see Table 1). However, compared to subjects who had failed antidepressant monotherapy, subjects who had failed combination therapy had longer episodes but a statistically higher rate of remission when treated with olanzapine monotherapy (see Table 2). Thus, differences in remission rates cannot be solely explained by differences in episode duration. Furthermore, because of the inherent unreliability of precisely defining the current episode duration, even with use of the SCID, we compared remission rates based on treatment during the previous six months with remission rates based on prior treatment for the entire episode and found very similar results. Another potential limitation is the relatively high drop-out rate across the groups (42.5%) and the observed differences in drop-out rates between the prior treatment groups. However, as expected, the drop-out rates are closely and inversely associated with the remission rates and are in keeping with our overall findings: the no prior treatment group (that had the highest remission rate) had the lowest drop-out rate and the adequate antidepressant group (that had the lowest remission rate) had the highest drop-out rate. The intermediate drop-out rate of patients who had failed combination therapy may also explain their intermediate rate of remission (i.e., having been able to tolerate an adequate combination of an antidepressant and an antipsychotic, they may be less likely to drop-out when they are treated with another antipsychotic and thus they may be more likely to remit).
Table 1.
Demographic and Clinical Characteristics of the Study Groups
| All N = 228 | No Prior treatment N = 31 | Inadequate prior treatment N = 94 | Adequate AD course N = 80 | Adequate combination course N = 23 | |
|---|---|---|---|---|---|
| Female sex | N (column %) | ||||
| 146 (64.0) | 19 (61.2) | 61 (64.9) | 53 (66.3) | 13 (56.5) | |
| Race | |||||
| White | 189 (82.9) | 26 (83.9) | 78 (83.0) | 68 (85.0) | 17 (73.9) |
| Black | 27 (11.8) | 3 (9.7) | 10 (10.6) | 10 (12.5) | 4 (17.4) |
| Asian | 12 (5.3) | 2 (6.5) | 6 (6.4) | 2 (2.5) | 2 (8.7) |
| Ethnicity | |||||
| Not Hispanic | 200 (87.7) | 26(83.9) | 81 (86.2) | 73 (91.3) | 20 (87.0) |
| Hispanic | 28 (12.3) | 5(16.1) | 13 (13.8) | 7 (8.8) | 3 (13.0) |
| Recruited on inpatient unit | 164 | 19 (61.3) | 80 (85.1) | 54 (67.5) | 11 (47.8) |
| Drop-Outs | 97 (42.5) | 7 (22.6) | 39 (41.5) | 43 (53.8) | 8 (34.8) |
| Mean (SD) | |||||
| Age | 51.5 (17.5) | 57.5 (20.1) | 61.8 (16.2) | 53.4 (14.3) | |
| MMSE | 27.1 (3.4) | 27.7 (2.3) | 27.3(3.4) | 27.2 (3.1) | |
| Duration of current Episode (months) | 7.6 (8.8) | 6.2 (7.1) | 13.4 (24.9) | 23.0 (34.0) | |
| Time in the study trial (weeks) | 8.8 (4.2) | 9.9 (4.1) | 8.6 (4.4) | 8.3 (4.1) | 9.7 (3.9) |
| HDRS-17* - Baseline | 29.8 (6.0) | 30.2 (5.1) | 29.5 (5.3) | 29.4 (5.6) | |
| Post | 11.9 (9.6) | 13.0 (10.7) | 17.6 (8.9) | 17.1 (11.0) | |
| Change | 17.9 (10.7) | 17.3 (10.2) | 11.9 (8.3) | 12.2 (8.1) | |
| % Change | 59.6 (32.1) | 57.9 (32.7) | 40.4 (26.3) | 44.2 (30.8) | |
| HDRS-24*- Baseline | 40.9 (8.5) | 40.9 (8.0) | 40.1 (7.9) | 40.6 (8.5) | |
| Post | 15.4 (13.1) | 17.4 (14.8) | 24.1 (12.2) | 23.4 (15.1) | |
| Change | 25.6 (14.2) | 23.5 (14.8) | 15.9 (11.7) | 17.2 (11.2) | |
| % Change | 62.4 (31.9) | 57.8 (33.7) | 39.8 (27.4) | 45.1 (30.1) | |
| BPRS – Baseline | 54.9 (11.4) | 55.6 (10.4) | 54.1 (9.9) | 57.7 (8.7) | |
| Post | 30.5 (13.5) | 32.4 (14.9) | 38.4 (14.7) | 38.6 (14.6) | |
| Change | 24.4 (14.4) | 23.2 (16.8) | 15.6 (13.1) | 19.2 (15.2) | |
| % Change | 43.9 (20.8) | 40.7 (26.3) | 28.9 (22.3) | 32.5 (26.8) | |
SD: standard deviation; CIRS-G: Cumulative Illness Rating Scale for Geriatrics; MMSE: Mini Mental State Examination; HDRS-17: 17-item version of the Hamilton Depression Rating Scale;
HDRS-24: 24-item version of the Hamilton Depression Rating Scale; BPRS: Brief Psychiatric Rating Scale
One participant refused to answer an item; that missing item score was prorated.
Additional studies are needed to confirm our findings and to assess the impact of prior courses of pharmacotherapy on treatment outcomes, not only in the short-term but also in the longer term. Nevertheless, our results emphasize the importance of characterizing the history of prior treatment when considering various treatment options in patients with MDpsy.
Acknowledgments
None.
Grants Supported by USPHS grants MH 62446, MH 62518, MH 62565, MH 62624, MH069430 and MH 086686 from the National Institute of Mental Health and by a fellowship from the Canadian Institutes of Health Research - Institute of Aging (DMB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration and URL: Clinicaltrials.gov
Registry ID: NCT00056472
References
- Amsterdam JD, Williams D, Michelson D, Adler LA, Dunner DL, Nierenberg AA, Reimherr FW, Schatzberg AF. Tachyphylaxis after repeated antidepressant drug exposure in patients with recurrent major depressive disorder. Neuropsychobiology. 2009;59:227–33. doi: 10.1159/000226611. [DOI] [PubMed] [Google Scholar]
- Andreescu C, Mulsant BH, Peasley-Miklus C, Rothschild AJ, Flint AJ, Heo M, Caswell M, Whyte EM, Meyers BS. Persisting low use of antipsychotics in the treatment of major depressive disorder with psychotic features. J Clin Psychiatry. 2007;68:194–200. doi: 10.4088/jcp.v68n0203. [DOI] [PubMed] [Google Scholar]
- Arean PA, Raue P, Mackin RS, Kanellopoulos D, McCulloch C, Alexopoulos GS. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction. Am J Psychiatry. 2010;167:1391–8. doi: 10.1176/appi.ajp.2010.09091327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell W, Leon A, Winokur G, Endicott J, Keller M, Akiskal H, Solomon D. Importance of psychotic features to long-term course in major depressive disorder. Am J Psychiatry. 1996;153:483–9. doi: 10.1176/ajp.153.4.483. [DOI] [PubMed] [Google Scholar]
- Davis JM, Chen N. Dose response and dose equivalence of antipsychotics. J Clin Psychopharmacol. 2004;24:192–208. doi: 10.1097/01.jcp.0000117422.05703.ae. [DOI] [PubMed] [Google Scholar]
- Dombrovski AY, Mulsant BH, Haskett RF, Prudic J, Begley AE, Sackeim HA. Predictors of remission after electroconvulsive therapy in unipolar major depression. J Clin Psychiatry. 2005;66:1043–9. doi: 10.4088/jcp.v66n0813. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35:837–44. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- First MB, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State - Practical Method for Grading Cognitive State of Patients for Clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU assessment manual for psychopharmacology, revised. Department of Health, Education, and Welfare; Rockville, Md.: 1976. pp. Document no. ADM 76-338. [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennings JM, Owashi T, Binder EB, Horstmann S, Menke A, Kloiber S, Dose T, Wollweber B, Spieler D, Messer T, Lutz R, Kunzel H, Bierner T, Pollmacher T, Pfister H, Nickel T, Sonntag A, Uhr M, Ising M, Holsboer F, Lucae S. Clinical characteristics and treatment outcome in a representative sample of depressed inpatients - findings from the Munich Antidepressant Response Signature (MARS) project. J Psychiatr Res. 2009;43:215–29. doi: 10.1016/j.jpsychires.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Kellner CH, Knapp RG, Petrides G, Rummans TA, Husain MM, Rasmussen K, Mueller M, Bernstein HJ, O'Connor K, Smith G, Biggs M, Bailine SH, Malur C, Yim E, McClintock S, Sampson S, Fink M. Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression - A multisite study from the Consortium for Research in Electroconvulsive Therapy (CORE) Archives of General Psychiatry. 2006;63:1337–1344. doi: 10.1001/archpsyc.63.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Haas G, Miewald J, Montrose DM, Reddy R, Schooler NR, Sweeney JA. Prolonged untreated illness duration from prodromal onset predicts outcome in first episode psychoses. Schizophr Bull. 2003;29:757–69. doi: 10.1093/oxfordjournals.schbul.a007045. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The Epidemiology of Major Depressive Disorder: Results From the National Comorbidity Survey Replication (NCS-R) JAMA: The Journal of the American Medical Association. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ. Achieving adequate outcomes in geriatric depression: standardized criteria for remission. J Clin Psychopharmacol. 2005;25:S24–8. doi: 10.1097/01.jcp.0000168488.99268.e5. [DOI] [PubMed] [Google Scholar]
- Meyers BS, English J, Gabriele M, Peasley-Miklus C, Heo M, Flint AJ, Mulsant BH, Rothschild AJ. A delusion assessment scale for psychotic major depression: Reliability, validity, and utility. Biol Psychiatry. 2006;60:1336–42. doi: 10.1016/j.biopsych.2006.05.033. [DOI] [PubMed] [Google Scholar]
- Meyers BS, Flint AJ, Rothschild AJ, Mulsant BH, Whyte EM, Peasley-Miklus C, Papademetriou E, Leon AC, Heo M. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD) Arch Gen Psychiatry. 2009;66:838–47. doi: 10.1001/archgenpsychiatry.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF., 3rd Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–48. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Price LH, Jatlow PI. Neuroleptic dose and desipramine concentrations during combined treatment of unipolar delusional depression. Am J Psychiatry. 1986;143:1151–4. doi: 10.1176/ajp.143.9.1151. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Baca-Garcia E, Kartachov A, Khait V, Campbell CE, Richards M, Sackeim HA, Prudic J, Mann JJ. A computer algorithm for calculating the adequacy of antidepressant treatment in unipolar and bipolar depression. J Clin Psychiatry. 2003;64:825–33. doi: 10.4088/jcp.v64n0714. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Malone KM, Ellis SP, Sackeim HA, Mann JJ. Inadequacy of antidepressant treatment for patients with major depression who are at risk for suicidal behavior. Am J Psychiatry. 1999;156:190–4. doi: 10.1176/ajp.156.2.190. [DOI] [PubMed] [Google Scholar]
- Patten SB, Wang JL, Williams JV, Currie S, Beck CA, Maxwell CJ, El-Guebaly N. Descriptive epidemiology of major depression in Canada. Can J Psychiatry. 2006;51:84–90. doi: 10.1177/070674370605100204. [DOI] [PubMed] [Google Scholar]
- Petrides G, Fink M, Husain MM, Knapp RG, Rush AJ, Mueller M, Rummans TA, O'Connor KM, Rasmussen KG, Jr., Bernstein HJ, Biggs M, Bailine SH, Kellner CH. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT. 2001;17:244–53. doi: 10.1097/00124509-200112000-00003. [DOI] [PubMed] [Google Scholar]
- Prudic J, Haskett RF, Mulsant B, Malone KM, Pettinati HM, Stephens S, Greenberg R, Rifas SL, Sackeim HA. Resistance to antidepressant medications and short-term clinical response to ECT. American Journal of Psychiatry. 1996;153:985–992. doi: 10.1176/ajp.153.8.985. [DOI] [PubMed] [Google Scholar]
- Rasmussen KG, Mueller M, Knapp RG, Husain MM, Rummans TA, Sampson SM, O'Connor MK, Petrides G, Fink M, Kellner CH. Antidepressant medication treatment failure does not predict lower remission with ECT for major depressive disorder: a report from the consortium for research in electroconvulsive therapy. J Clin Psychiatry. 2007;68:1701–6. doi: 10.4088/jcp.v68n1109. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, 3rd, Frank E, Kupfer DJ, Thase ME, Perel JM, Mazumdar S, Houck PR. Treatment outcome in recurrent major depression: a post hoc comparison of elderly (“young old”) and midlife patients. Am J Psychiatry. 1996;153:1288–92. doi: 10.1176/ajp.153.10.1288. [DOI] [PubMed] [Google Scholar]
- Roose SP, Sackeim HA, Krishnan KRR, Pollock BG, Alexopoulos G, Lavretsky H, Katz IR, Hakkarainen H, Old-Old Depression Study, G. Antidepressant Pharmacotherapy in the Treatment of Depression in the Very Old: A Randomized, Placebo-Controlled Trial. American Journal of Psychiatry. 2004;161:2050–2059. doi: 10.1176/appi.ajp.161.11.2050. [DOI] [PubMed] [Google Scholar]
- Rothschild AJ. Challenges in the treatment of depression with psychotic features. Biol Psychiatry. 2003;53:680–90. doi: 10.1016/s0006-3223(02)01747-x. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Haskett RF, Mulsant BH, Thase ME, Mann JJ, Pettinati HM, Greenberg RM, Crowe RR, Cooper TB, Prudic J. Continuation Pharmacotherapy in the Prevention of Relapse Following Electroconvulsive Therapy: A Randomized Controlled Trial. JAMA: The Journal of the American Medical Association. 2001;285:1299–1307. doi: 10.1001/jama.285.10.1299. [DOI] [PubMed] [Google Scholar]
- Schimmelmann BG, Huber CG, Lambert M, Cotton S, McGorry PD, Conus P. Impact of duration of untreated psychosis on pre-treatment, baseline, and outcome characteristics in an epidemiological first-episode psychosis cohort. J Psychiatr Res. 2008;42:982–90. doi: 10.1016/j.jpsychires.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Spiker DG, Weiss JC, Dealy RS, Griffin SJ, Hanin I, Neil JF, Perel JM, Rossi AJ, Soloff PH. The pharmacological treatment of delusional depression. Am J Psychiatry. 1985;142:430–6. doi: 10.1176/ajp.142.4.430. [DOI] [PubMed] [Google Scholar]
- Tew JD, Mulsant BH, Houck PR, Lenze E, Whyte EM, Miller MD, Stack JA, Bensasi S, Reynolds CF. Impact of prior treatment exposure on response to antidepressant treatment in late life. American Journal of Geriatric Psychiatry. 2006;14:957–965. doi: 10.1097/01.JGP.0000222311.70424.85. [DOI] [PubMed] [Google Scholar]
- Williams JB, Kobak KA, Bech P, Engelhardt N, Evans K, Lipsitz J, Olin J, Pearson J, Kalali A. The GRID-HAMD: standardization of the Hamilton Depression Rating Scale. Int Clin Psychopharmacol. 2008;23:120–9. doi: 10.1097/YIC.0b013e3282f948f5. [DOI] [PubMed] [Google Scholar]