Table 2.
Reaction scope of homodimerizationa
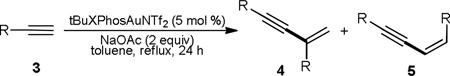 | ||||
|---|---|---|---|---|
| entry | R | 4 | yield (4)b | 4/5c |
| 1 | n-decyl | 4b | 72% | 14/1 |
| 2 | cyclohexyl | 4c | 69% | 13/1 |
| 3d | cyclopentyl | 4d | 85% | 25/1 |
| 4d | 4e | 61% | 12/1 | |
| 5 | PhCH2CH2 | 4f | 61% | 11/1 |
| 6 | 4g | 78% | 10/1 | |
| 7 | 4h | 81% | 11/1 | |
| 8 | Ph | 4i | 8% | 12/1 |
| 9 | 2-propenyl | 4j | - | - |
Reaction run in Schlenk tubes under nitrogen; [alkyne] = 0.1 M.
Isolated yield and containing small amount of inseparable 5.
Determined by crude 1H NMR.
Reaction time: 48 h; catalyst loading: 10 mol %.
