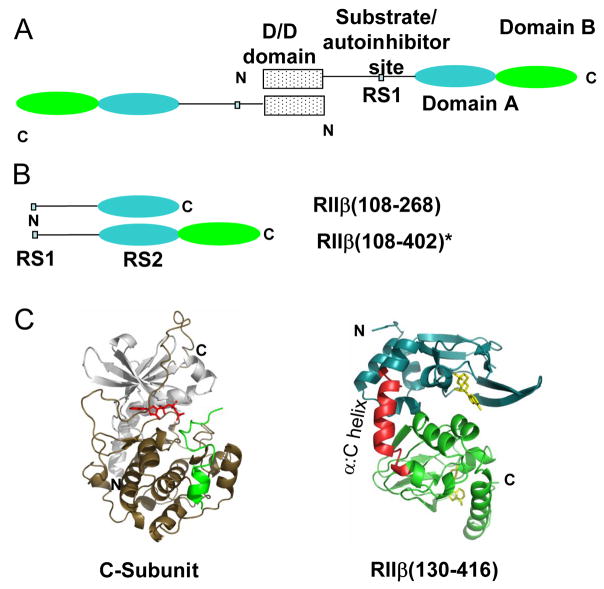
Figure 1.
Type II PKA. (A) Domain organization of PKA RIIβ with an N-terminal dimerization/docking domain (D/D domain), substrate/autoinhibitor site, and two cAMP binding domains, Domain A (108–268) and Domain B (269–416). (B) Deletion mutants of RIIβ for probing interactions with the C-subunit, RIIβ(108–268) and RIIβ(108–402). * Increased proteolytic cleavage of C-terminal residues 403-416 during purification of the RIIβ(108–402):C holoenzyme, necessitated use of a more stable, double truncation mutant RIIβ(108–402) for studying interactions with the C-subunit. (C) Structures of the catalytic subunit of PKA (left) (PDB access code 1ATP) [38] and of RIIβ(right) (PDB access code 1CX4) [35]. The inhibitor peptide is green and ATP is red for C-subunit (left). In RIIβ(right), residues 108-252 are in blue, while residues 269-416 are green and the two cAMP molecules are yellow. The α:C helix (residues 253-268), a hotspot for interactions with the C-subunit is in red [17].