Abstract
Background
Multi-detector cardiac computed tomography (CT) allows for simultaneous assessment of aortic distensibility (AD), coronary atherosclerosis, and thoracic aortic atherosclerosis.
Objectives
We sought to determine the relationship of AD to the presence and morphological features in coronary and thoracic atherosclerosis.
Methods
In 293 patients (53±12 years, 63% male), retrospectively-gated MDCT were performed. We measured intraluminal aortic areas across 10 phases of the cardiac cycle (multiphase reformation 10% increments) at pre-defined locations to calculate the ascending, descending, and local AD (at locations of thoracic plaque). AD was calculated as maximum change in area/(minimum area × pulse pressure). Coronary and thoracic plaques were categorized as calcified, mixed, or non-calcified.
Results
Ascending and descending AD were lower in patients with any coronary plaque, calcified or mixed plaque than those without (all p<0.0001) but not with non-calcified coronary plaque (p≥0.46). Per 1 mmHg−110−3 increase in ascending and descending AD, there was an 18–29% adjusted risk reduction for having any coronary, calcified plaque, or mixed coronary plaque (ascending AD only) (all p≤0.04). AD was not associated with non-calcified coronary plaque or when age was added to the models (all p>0.39). Local AD was lower at locations of calcified and mixed thoracic plaque when compared to non-calcified thoracic atherosclerosis (p<0.04).
Conclusions
A stiffer, less distensible aorta is associated with coronary and thoracic atherosclerosis, particularly in the presence of calcified and mixed plaques, suggesting that the mechanism of atherosclerosis in small and large vessels is similar and influenced by advancing age.
Keywords: aortic distensibility, coronary atherosclerosis, thoracic atherosclerosis, peripheral vascular disease, computed tomography, cardiovascular aging
1. Introduction
Aortic distensibility (AD), or the degree to which the aorta can contract and expand, is a property of the vessel wall itself. It is defined as the maximum change in area/(minimum area × pulse pressure). AD can be measured at any point along the aorta, and previous studies have confirmed changes in distensibility in both the ascending and descending aorta.[1–2] AD has been shown to be inversely proportional to disease severity and presence of cardiovascular risk factors.[3] It is markedly decreased in patients with known coronary artery disease (CAD) and is inversely proportional to the amount of CAD as determined by the degree of stenosis on invasive coronary angiography.[4–5] AD using ultrasound on medium-caliber vessels, specifically the carotid artery, is feasible due to its superficial location.[6]
With both improved temporal and spatial resolution, 64-slice contrast-enhanced multidetector computed tomography (MDCT) with electrocardiogram-gating allows for accurate visualization of small and large-caliber vasculature and their contractility throughout to the cardiac cycle.[7–8] This advance has made possible the measurement of AD by MDCT.[9–10] Furthermore, MDCT can simultaneously characterize coronary and thoracic aortic atherosclerotic plaque morphology by delineating between calcified, mixed, non-calcified lesions.[7, 11–12]
Currently there is paucity of data relating MDCT-measured AD to coronary and thoracic plaque morphology as a single imaging modality. To better understand the mechanism between AD and plaque morphology in small and large caliber vessels, we sought (1) to determine the association of AD to the presence and type of coronary atherosclerosis and (2) examine the relationship of local AD to thoracic atherosclerosis plaque using MDCT.
2. Materials and Methods
2.1 Study population
In this substudy of the “Rule Out Myocardial Infarction using Computer Assisted Tomography” (ROMICAT) trial,[13] acute chest pain patients from the emergency department with a low-to-intermediate likelihood of acute coronary syndrome were enrolled between May 2005 and May 2007. The details of the study design and results have been described previously[13] and was supported by NIH (R01 HL080053). Briefly, 368 patients underwent ECG-gated contrast-enhanced MDCT for coronary artery assessment but the results remained blinded to both the patients and the caregivers. The institutional review board of our institution approved the study protocol and all patients provided written informed consent. The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology.[14]
In this analysis, we excluded 51 patients because the scan range did not include the level of the bifurcation of the pulmonary trunk in the CT multi-phase reconstruction (MPR) datasets, which is needed for our ascending AD measurement. We excluded an additional 24 patients due to missing systolic and diastolic blood pressure recordings at the time of the MDCT image acquisition. A total of 293 patients were included in this analysis.
2.2 MDCT Imaging Protocol
MDCT was performed using a standard coronary artery imaging protocol on a 64-slice MDCT (Sensation 64, Siemens Medical Solutions, Forchheim, Germany) that included the administration of 0.6 mg sublingual nitroglycerin and intravenous beta-blocker (5–20 mg intravenous metoprolol) if the resting heart rate was above 60 beats per minute and if no contraindications were present.
Images were acquired during a single breath hold at inspiration in spiral mode with 330 ms rotation time, 32 × 0.6 mm collimation, a tube voltage of 120 kVp, and maximum effective tube current-time product of 850 mAs. ECG-tube modulation was used when possible. A test bolus protocol was used to determine optimal contrast enhancement of the coronary arteries (20 ml contrast agent followed by 40 ml saline, flow rate of 5 ml/s). Contrast agent (80–100 ml, Iodhexodol 320 g/cm3, Visipaque, General Electrics Healthcare, Princeton, NJ, USA) with 40 ml saline was injected intravenously at a rate of 5 ml/s during acquisition of images. Reconstructions were made using retrospectively ECG-gated half-scan algorithm allowing a temporal resolution of 165 ms.
For AD measurements, multiphase reformation (MPR) dataset was reconstructed at 10% RR interval (10 phases) with 1.5 mm slice thickness and 1.5 mm increments of the transaxial images. For coronary and aortic plaque evaluation, 3 transaxial datasets per subject, on average, were reconstructed with a 512 × 512 pixel image matrix, an increment of 0.4 mm and a slice thickness of 0.75 mm.
2.3 AD Measurements
AD was calculated as maximum change in area/(minimum area × pulse pressure).[9] Pulse pressure was calculated from blood pressure measurements recorded in the supine position immediately after MDCT scan acquisition. We performed measurements for the ascending AD, descending AD, and local AD. The aortic lumen was measured at each of the 10 phases of the cardiac cycle (10% increments of the RR interval, Figure 1). For the ascending AD, measurements were taken at the level of the bifurcation of the pulmonary trunk; and for the descending AD, they were taken at the diaphragm at the lowest level present in all scans, as previously described.[15] For the determination of the relationship of thoracic atherosclerosis and local AD, measurements were made intraluminally at the level of the thoracic plaque.
Figure 1. Method of measuring aortic distensibility.
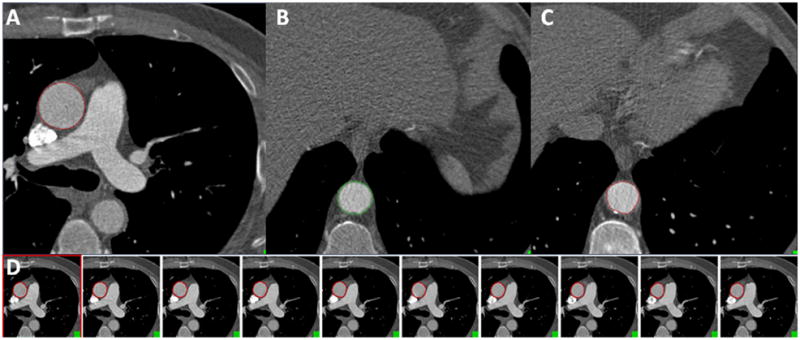
(A) For ascending AD, the ascending aortic diameter (red circle) was measured at the level of the bifurcation of the pulmonary arteries. (B) For descending AD, the descending aortic diameter (green circle) was measured at the level of the diaphragm. (C) For local AD, the aortic intraluminal diameter (red circle) was measured at the location of a segment with calcified thoracic aortic plaque. (D) An example depicting ascending AD measurements of the aortic areas measured across serial transaxial slices at 10% increments of the cardiac cycle at the level of the bifurcation of the pulmonary arteries.
Two CT readers performed the measurements on a dedicated offline workstation (QMass CT v 7.1, Medis Medical Imaging Systems, Leiden, the Netherlands). The inter-observer reproducibility for the AD measures was determined for 20 randomly selected studies using intraclass correlation coefficient (ICC). Since the formula for calculating AD includes both the maximum and minimum vessel area, ICC was performed on those measures in both the ascending aorta and the descending aorta. The inter-observer ICC for the maximum area of the ascending aorta was 0.983 and for the descending aorta was 0.981 (all p<0.0001). The inter-observer ICC for minimum vessel area for the ascending aorta was 0.988 and was the same for the descending aorta (all p<0.0001). The intra-observer ICC for maximum and minimum ascending aorta area and descending aorta area were excellent (ICC: 0.981, 0.972, 0.967 and 0.973, respectively; all p<0.0001).
2.4 Coronary Artery and Thoracic Aortic Plaque Assessment
For coronary atherosclerosis morphology, the presence of calcified, mixed, and non-calcified plaque were assessed for each patient using the modified American Heart Association classification with 17-segments.[16] Plaque detection and characterization by MDCT has been described and validated elsewhere.[13] Briefly, calcified plaque was determined to be present if a structure with a density of >130 Hounsfield units (HU) was visualized separate from the contrast-enhanced coronary lumen, was assigned to the coronary wall, and was identified in at least 2 independent planes. Non-calcified plaque was defined as any clearly visible structure that could be assigned to the coronary artery wall in at least 2 independent planes and had a density of <130 HU but had a greater density than the surrounding tissue. Segments were determined to have mixed plaque if there were calcified and non-calcified plaque components within one coronary artery segment.
For thoracic aortic plaque assessment, we determined the presence of thoracic atherosclerosis at the pre-defined levels of ascending and descending AD measurements. For local AD analysis, we evaluated for thoracic atherosclerotic morphology (calcified, mixed, or non-calcified) on transaxial images. Thoracic aortic plaques were confirmed in 2 or more phases and all separate plaques were included in the analysis.
2.5 Covariates of Interest
Assessment of patients’ medical history and cardiovascular risk factors was completed at the time of enrollment based on medical records and self-report. Body mass index (BMI) was defined as weight (kilograms) divided by the height squared (meters). Diabetes mellitus was defined as a fasting plasma glucose ≥126 mg/dL or treatment with a hypoglycemic agent. Patients were defined as having a history of hypertension if they were currently taking anti-hypertensive medications. Hyperlipidemia was defined as total cholesterol of ≥200 mg/dl or treatment with a lipid lowering medication. Patients were defined as being smokers if they smoke at least 1 cigarette a day currently or in the past. Family history of CAD was defined as having a first-degree female (<65 years) or male (<55 years) relative with a documented history of myocardial infarction or sudden cardiac death. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) formula as described previously.[17]
2.6 Statistical Analysis
Descriptive statistics were expressed as mean ± standard deviation (SD) or median with interquartile range (IQR) for continuous variables and as frequency and percentages for nominal variables, as appropriate. We used Pearson’s correlation to compare normally distributed continuous variables and Student’s t-test for comparing normally distributed covariates. We used logistic regression to determine the association between AD and coronary atherosclerosis. For the multivariable models, we determined the association between ascending AD and descending AD with the presence and morphology of coronary plaque (any, calcified, mixed, and non-calcified) using logistic regression models with and without age. In the first model (without age), we adjusted for covariates of interest from Table 1 (p≤0.10) including gender, diabetes, hypertension, hyperlipidemia, family history of CAD, eGFR, and the presence of thoracic aortic plaque at the level of ascending or descending AD measurement. The second model adjusted for the same variables but included age. To determine the relationship of aortic plaque morphology on local AD, we used a mixed effect model which accounted for the within subject variability. A 2-tailed p-value of <0.05 was considered to indicate statistical significance. All analyses were performed using SAS (Version 9.2, SAS Institute Inc., Cary, North Carolina) and SPSS (Version 16.0, SPSS Inc., Chicago, Illinois).
Table 1.
Patient demographics and covariates of interest in the overall cohort and as stratified by patients with or without CAD.
| Overall Cohort (n=293) | Patients with CAD (n=152) | Patients without CAD (n=141) | p-value | |
|---|---|---|---|---|
| Age, yrs | 53.0 ± 12.0 | 58.4 ± 11.7 | 47.2 ± 9.2 | <0.0001 |
| Male | 183 (62.5%) | 102 (67.1%) | 81 (57.5%) | 0.09 |
| BMI, kg/m2 | 29.3 ± 6.2 | 29.7 ± 6.2 | 29.0 ± 6.2 | 0.33 |
| Diabetes | 33 (11.3%) | 22 (14.5%) | 11 (7.8%) | 0.07 |
| Hypertension | 124 (42.3%) | 87 (57.2%) | 37 (26.2%) | <0.0001 |
| Hyperlipidemia | 110 (37.5%) | 79 (52.0%) | 31 (22.0%) | <0.0001 |
| Smoking | 147 (50.2%) | 92 (60.5%) | 55 (39.0%) | 0.0002 |
| Family History of CAD | 76 (26.0%) | 47 (30.9%) | 29 (20.1%) | 0.05 |
| eGFR, ml/min/1.73m2 | 84.2 ± 18.0 | 81.3 ± 17.7 | 87.3 ± 17.9 | 0.004 |
| Thoracic plaque at level of ascending AD | 10 (3.4%) | 8 (5.3%) | 2 (1.4%) | 0.10 |
| Thoracic plaque at level of descending AD | 49 (16.7%) | 33 (21.7%) | 16 (11.4%) | 0.02 |
CAD denotes coronary artery disease; BMI, body mass index; eGFR, estimated glomerular filtration rate; and AD, aortic distensibility.
3. RESULTS
3.1 Baseline characteristics
The patient demographics of the study cohort are summarized in Table 1. In this cohort of 293 patients, the average age was 53±12 years and 62.5% of patients were men. Patients with CAD were more likely to smoke, have hyperlipidemia and hypertension, but lower eGFR. There was a trend towards higher incidence of diabetes, and family history of CAD in patients with CAD.
3.2 Correlations of Ascending and Descending Aortic Distensibility
Ascending AD for the entire cohort was 2.9 ± 1.6 mmHg−110−3 and descending AD was 3.1 ± 1.5 mmHg−110−3. Both ascending and descending AD were positively correlated (r=0.64, p<0.0001). There were no gender-based differences in AD (ascending AD: 2.9±1.6 vs. 2.6±1.8 mmHg−110−3, p=0.15; descending AD: 3.1±1.4 vs. 3.0±1.6 mmHg−110−3, p=0.47; for men and women, respectively). Both were negatively correlated to systolic blood pressure (ascending AD: r=−0.41, p<0.0001; descending AD: r=−0.46, p<0.0001) but not with diastolic blood pressure (both p≥0.48). Importantly, there was a negative correlation between age and AD (ascending AD: r=−0.53, p<0.0001; descending AD: r=−0.51, p<0.0001).
3.3 Association between Aortic Distensibility and Coronary Atherosclerosis
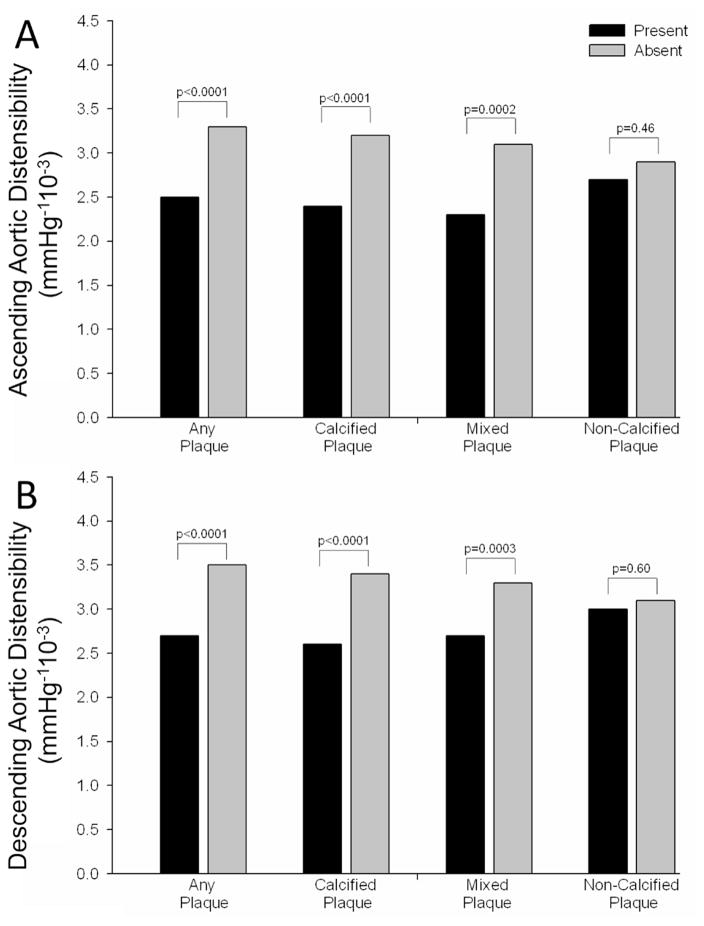
Out of the 293 patients, 152 (51.9%) patients had plaque in their coronary arteries, 120 (41.0%) had calcified plaque, 92 (31.4%) had mixed plaque, and 47 (16.0%) had non-calcified plaque. As shown in Figure 2, patients with any coronary plaque, calcified plaque, or mixed plaque had significantly lower ascending and descending AD than those without (all p≤0.0002). However, there was no difference in either AD in patients with non-calcified coronary plaque as compared to those without (both p≥0.46).
Figure 2.
Differences in ascending (A) and descending (B) aortic distensibility in patients with and without any, calcified, mixed, and non-calcified coronary plaque.
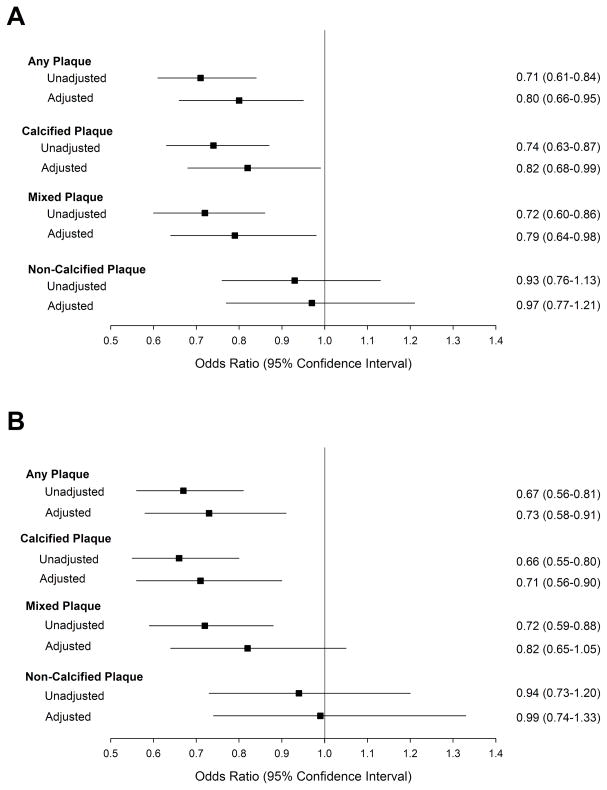
In adjusted analysis (Figure 3A), a 1 mmHg−110−3 increase in ascending AD was associated with an 18–21% reduced risk for the presence of any coronary plaque, calcified plaque, and mixed plaque (all p≤0.03) but not with non-calcified plaque (p=0.77). For descending AD (Figure 3B), a 1 mmHg−110−3 increase was associated with a 27–29% reduction in risk for the presence of any CAD and calcified plaque (both p<0.005) but not significant with mixed or non-calcified plaque (both p>0.11). These associations between AD and coronary plaque were no longer significant after adding age to the multivariable models (all p-value>0.39, data not shown).
Figure 3. Unadjusted and adjusted analyses of the association between ascending (A) and descending (B) aortic distensibility for the presence of any, calcified, mixed and non-calcified coronary plaque.
Multivariable models adjusted for gender, diabetes, hypertension, hyperlipidemia, family history of CAD, eGFR, and the presence of local ascending or descending aortic plaque. Abbreviations as in Table 1.
3.4 Relation between Local AD and Thoracic Atherosclerosis
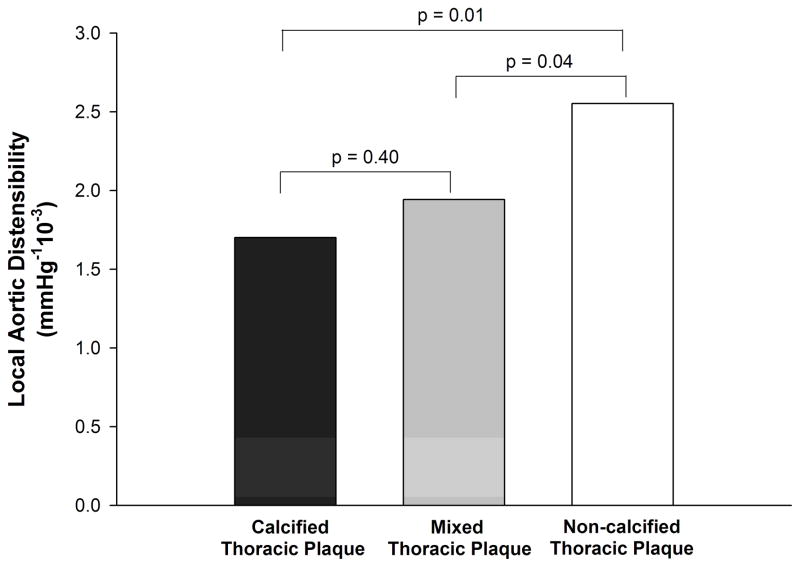
There were 73 (24.9%) patients with thoracic aortic atherosclerosis, of which there were 21 calcified, 23 mixed, and 40 non-calcified aortic plaques. As shown in Figure 4, local AD was lower in the location of calcified thoracic aortic plaque as compared to that of non-calcified plaque (1.7 vs. 2.6 mmHg−110−3, p=0.01). Similarly, local AD was lower in regions of mixed thoracic aortic plaque as compared to regions of non-calcified plaque (1.9 vs. 2.6 mmHg−110−3, p=0.04). No difference was seen in local AD between calcified and mixed aortic plaque (1.7 vs. 1.9 mmHg−110−3, p=0.40).
Figure 4.
Local aortic distensibility at the level of calcified, mixed, and non-calcified thoracic aortic atherosclerosis.
4. DISCUSSION
In this study we describe the relationship between AD and coronary and thoracic atherosclerosis based on ECG-gated contrast-enhanced MDCT in a large cohort of patients presenting with acute chest pain without a known history of coronary artery disease. The strength of our study is the simultaneous acquisition of the AD measurements (ascending, descending, and local AD) as well as characterization of coronary and thoracic aortic plaque morphology using a single imaging modality. In addition to a positive correlation between ascending and descending AD, there was a negative correlation between age and AD. In small caliber vessels, we found that ascending and descending AD were lower in patients with more advanced stages of CT detectable coronary atherosclerosis, specifically calcified and mixed plaque but not with non-calcified plaque. Importantly, these associations were driven by age. Furthermore, in large caliber vessels, we found that local AD was lower at the regions of calcified and mixed thoracic aortic plaque as compared to non-calcified plaque.
4.1 Aortic Distensibility in Relation to Coronary Calcification and Age
The presence of calcification is a significant contributor to increased arterial stiffness and thus reduced AD [18]. Our study supports this relationship between age, vascular calcification and increased vascular stiffness. This is not unexpected as patients with calcified coronary plaque are older[19], as are patients with mixed plaque compared to those with either no disease or exclusively non-calcified plaque.[20] The presence and extent of vascular calcification has also been shown to be a strong predictor of vascular morbidity and mortality in the general population.[21] Our study supports these findings that arterial calcification may be thought of as a sign of vascular aging.
Calcification in the arterial vasculature has been proposed to occur at the later stage of the atherosclerosis process.[22] Increased production and irreversible cross-linking of collagen fibers, reduced levels of elastin, infiltration of vascular smooth muscle cells, macrophages and mononuclear cells, and increased matrix metalloproteinases are the sequelae of vascular inflammation which contribute to reduced arterial distensibility.[23] As calcification takes time to develop,[24–25] it is not surprising that increasing age is a strong confounder in our adjusted analyses and the driving force for increased arterial stiffness and the atherosclerosis process.
4.2 Local Aortic Distensibility and Thoracic Plaque Morphology
The presence of thoracic aortic calcification by non-contrast enhanced CT scans has been shown to be independently associated with reduced ascending thoracic AD measured by magnetic resonance imaging in a general population free of known cardiovascular disease. [26] Our findings confirm this relationship between calcified aortic plaque and reduced AD, however the strength of our study lies in the use of a single imaging modality of contrast-enhanced CT. We were able to demonstrate that thoracic AD was reduced locally at the site of thoracic atherosclerosis, specifically at sites of calcified and mixed thoracic aortic plaque as compared to region of non-calcified plaque.
4.3 Arterial Mechanics of Different Vascular Beds
Our study examined not only the local thoracic AD at the site of thoracic atherosclerosis, it allowed for simultaneous assessment of coronary atherosclerosis and coronary plaque composition for better mechanistic understanding to the systemic atherosclerosis process of different vascular beds. We found that both ascending and descending AD were reduced by the presence of calcified and mixed coronary and thoracic atherosclerosis, which implies an interaction in arterial mechanics between different vascular beds, specifically small and large vasculature. Hence disease in one vascular territory may give insight into the health of other vascular beds.
The presence of thoracic aortic calcification has also been shown to have a strong independent association with increased carotid stiffness, a medium caliber vessel.[27] Regardless of the location of atherosclerosis (coronary, carotid, or thoracic aorta), the presence of vascular atherosclerosis is associated with reduced AD and increased arterial stiffness has been associated with higher cardiovascular event rates in both the general population [28–29]and high-risk cohorts [30–32]. Furthermore, there is evidence that patients with polyvascular disease have worse cardiac outcomes.[33] Our findings suggest that the mechanism of arterial stiffness and its relation with arterial calcification of both small and large vascular beds acts in a similar process. This may have clinical implications and suggests that patients with calcification as a manifestation of their vascular arterial disease have increased overall arterial stiffness and vascular aging, and may warrant more intensive medical therapy for their vascular risk factors.
4.4 Limitations
There are several noteworthy limitations to our study. AD was measured by 64-slice MDCT with a temporal resolution of 165 ms. Newer technology CT scanners such as dual-source CT provide better temporal resolution of 75 ms and subsequently may improve AD measurements.[34] Although this study provided mechanistic insight regarding the relationship between AD and the presence and morphology of coronary and thoracic atherosclerosis, we are not advocating the use of cardiac CT to assess AD, since alternative non-ionizing radiation modalities are available for AD assessment.[6, 26] Our study was feasible because it was performed using cardiac CT with retrospective gating for assessment of coronary arteries and left ventricular function in an acute chest pain population as part of the ROMICAT trial.[13] Thus, radiation exposure inherent in the acquisition of CT images should preclude cardiac CT from being routinely performed solely for AD measurements.
5. Conclusion
A stiffer, less compliant, less distensible aorta is associated with coronary and thoracic atherosclerosis, specifically calcified and mixed plaque. AD is affected by changes in wall content, particularly in the presence of calcium. Our findings suggest that arterial mechanics and the atherosclerotic process in small and large vessels are similar and influenced by advancing age, inferring a potential mechanistic link between arterial mechanics and atherosclerosis of different vascular beds.
Acknowledgments
Sources of Funding: This work was supported by the NIH R01 HL080053, and in part supported by Siemens Medical Solutions and General Electrics Healthcare. Dr. Truong received support from NIH grant K23HL098370 and L30HL093896.
Footnotes
Disclosures: No conflicts of interest to be disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ganten M, Krautter U, Hosch W, Hansmann J, von Tengg-Kobligk H, Delorme S, et al. Age related changes of human aortic distensibility: evaluation with ECG-gated CT. Eur Radiol. 2007;17:701–8. doi: 10.1007/s00330-006-0309-z. [DOI] [PubMed] [Google Scholar]
- 2.Ganten MK, Krautter U, von Tengg-Kobligk H, Bockler D, Schumacher H, Stiller W, et al. Quantification of aortic distensibility in abdominal aortic aneurysm using ECG-gated multi-detector computed tomography. Eur Radiol. 2008;18:966–73. doi: 10.1007/s00330-007-0833-5. [DOI] [PubMed] [Google Scholar]
- 3.Malayeri AA, Natori S, Bahrami H, Bertoni AG, Kronmal R, Lima JA, et al. Relation of aortic wall thickness and distensibility to cardiovascular risk factors (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2008;102:491–6. doi: 10.1016/j.amjcard.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giannattasio C, Capra A, Facchetti R, Viscardi L, Bianchi F, Failla M, et al. Relationship between arterial distensibility and coronary atherosclerosis in angina patients. J Hypertens. 2007;25:593–8. doi: 10.1097/HJH.0b013e3280119012. [DOI] [PubMed] [Google Scholar]
- 5.Stefanadis C, Stratos C, Boudoulas H, Kourouklis C, Toutouzas P. Distensibility of the ascending aorta: comparison of invasive and non-invasive techniques in healthy men and in men with coronary artery disease. Eur Heart J. 1990;11:990–6. doi: 10.1093/oxfordjournals.eurheartj.a059639. [DOI] [PubMed] [Google Scholar]
- 6.Giannattasio C, Failla M, Emanuelli G, Grappiolo A, Boffi L, Corsi D, et al. Local effects of atherosclerotic plaque on arterial distensibility. Hypertension. 2001;38:1177–80. doi: 10.1161/hy1101.095994. [DOI] [PubMed] [Google Scholar]
- 7.Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724–32. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Raff GL, Gallagher MJ, O’Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46:552–7. doi: 10.1016/j.jacc.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 9.Ganten M, Boese JM, Leitermann D, Semmler W. Quantification of aortic elasticity: development and experimental validation of a method using computed tomography. Eur Radiol. 2005;15:2506–12. doi: 10.1007/s00330-005-2857-z. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Fletcher JG, Vrtiska TJ, Manduca A, Thompson JL, Raghavan ML, et al. Large-vessel distensibility measurement with electrocardiographically gated multidetector CT: phantom study and initial experience. Radiology. 2007;245:258–66. doi: 10.1148/radiol.2451060530. [DOI] [PubMed] [Google Scholar]
- 11.Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359:2324–36. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 12.Achenbach S, Moselewski F, Ropers D, Ferencik M, Hoffmann U, MacNeill B, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation. 2004;109:14–7. doi: 10.1161/01.CIR.0000111517.69230.0F. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009;53:1642–50. doi: 10.1016/j.jacc.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coats AJ, Shewan LG. Statement on authorship and publishing ethics in the international journal of cardiology. Int J Cardiol. 2011;153:239–40. doi: 10.1016/j.ijcard.2011.10.119. [DOI] [PubMed] [Google Scholar]
- 15.Nelson AJ, Worthley SG, Cameron JD, Willoughby SR, Piantadosi C, Carbone A, et al. Cardiovascular magnetic resonance-derived aortic distensibility: validation and observed regional differences in the elderly. J Hypertens. 2009;27:535–42. doi: 10.1097/hjh.0b013e32831e4599. [DOI] [PubMed] [Google Scholar]
- 16.Raff GL, Abidov A, Achenbach S, Berman DS, Boxt LM, Budoff MJ, et al. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2009;3:122–36. doi: 10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–7. doi: 10.1161/01.hyp.33.5.1111. [DOI] [PubMed] [Google Scholar]
- 19.Pundziute G, Schuijf JD, van Velzen JE, Jukema JW, van Werkhoven JM, Nucifora G, et al. Assessment with multi-slice computed tomography and gray-scale and virtual histology intravascular ultrasound of gender-specific differences in extent and composition of coronary atherosclerotic plaques in relation to age. Am J Cardiol. 2010;105:480–6. doi: 10.1016/j.amjcard.2009.09.054. [DOI] [PubMed] [Google Scholar]
- 20.Bamberg F, Dannemann N, Shapiro MD, Seneviratne SK, Ferencik M, Butler J, et al. Association between cardiovascular risk profiles and the presence and extent of different types of coronary atherosclerotic plaque as detected by multidetector computed tomography. Arterioscler Thromb Vasc Biol. 2008;28:568–74. doi: 10.1161/ATVBAHA.107.155010. [DOI] [PubMed] [Google Scholar]
- 21.Wilson PW, Kauppila LI, O’Donnell CJ, Kiel DP, Hannan M, Polak JM, et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–34. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 22.Dunphy MP, Freiman A, Larson SM, Strauss HW. Association of vascular 18F-FDG uptake with vascular calcification. J Nucl Med. 2005;46:1278–84. [PubMed] [Google Scholar]
- 23.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–43. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 24.Budoff MJ, Nasir K, McClelland RL, Detrano R, Wong N, Blumenthal RS, et al. Coronary calcium predicts events better with absolute calcium scores than age-sex-race/ethnicity percentiles: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2009;53:345–52. doi: 10.1016/j.jacc.2008.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wayhs R, Zelinger A, Raggi P. High coronary artery calcium scores pose an extremely elevated risk for hard events. J Am Coll Cardiol. 2002;39:225–30. doi: 10.1016/s0735-1097(01)01737-5. [DOI] [PubMed] [Google Scholar]
- 26.Al-Mallah MH, Nasir K, Katz R, Takasu J, Lima JA, Bluemke DA, et al. Thoracic aortic distensibility and thoracic aortic calcium (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2010;106:575–80. doi: 10.1016/j.amjcard.2010.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blaha MJ, Budoff MJ, Rivera JJ, Katz R, O’Leary DH, Polak JF, et al. Relationship of Carotid Distensibility and Thoracic Aorta Calcification. Multi-Ethnic Study of Atherosclerosis. Hypertension. 2009 doi: 10.1161/HYPERTENSIONAHA.109.138396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–70. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–11. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–5. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 31.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 32.Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–6. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- 33.Bhatt DL, Peterson ED, Harrington RA, Ou FS, Cannon CP, Gibson CM, et al. Prior polyvascular disease: risk factor for adverse ischaemic outcomes in acute coronary syndromes. Eur Heart J. 2009;30:1195–202. doi: 10.1093/eurheartj/ehp099. [DOI] [PubMed] [Google Scholar]
- 34.Alkadhi H, Stolzmann P, Desbiolles L, Baumueller S, Goetti R, Plass A, et al. Low-dose, 128-slice, dual-source CT coronary angiography: accuracy and radiation dose of the high-pitch and the step-and-shoot mode. Heart. 2010;96:933–8. doi: 10.1136/hrt.2009.189100. [DOI] [PubMed] [Google Scholar]