Abstract
Aims
To compare the descriptive epidemiology of overactive bladder (OAB) of presumed neurologic origin (NOAB) to OAB of non-neurologic origin (N-NOAB).
Methods
5,503 community-dwelling persons aged 30-79 were interviewed regarding urologic symptoms (2002-2005). NOAB was defined as symptoms of urgency and/or urgency incontinence among those with a self-reported history of health care provider diagnosed stroke (N=98), multiple sclerosis (N=21), or Parkinson’s disease (N=7). N-NOAB was defined identically but occurring among those not reporting neurologic disease (ND). Prevalence estimates were weighted to reflect sampling design; chi-square, Fisher’s exact, or t-tests were used to test differences. Urologic symptom interference was assessed using the Epstein scale, while the impact of urinary incontinence (UI) on health-related quality-of-life (HRQOL) was measured using a modification of the Incontinence Impact Questionnaire-7.
Results
45 (31.0%) of 125 persons with ND and 994 (16.7%) of 5378 persons without ND reported OAB symptoms. The overall prevalence of NOAB and N-NOAB was 0.6% and 16.4%, respectively. Persons with NOAB had higher (worse) mean American Urologic Association Symptom Index scores (13.0 vs. 10.0, p=0.09) compared to those with N-NOAB, and were significantly more likely to have diabetes, high blood pressure, cardiac disease and fair/poor self-reported health (all p<0.05). Mean symptom interference and UI HRQOL scores were significantly higher (worse) in the NOAB group compared to persons with N-NOAB (all p<0.05).
Conclusions
Persons with NOAB appeared to have a greater burden of urologic illness with respect to symptom interference and HRQOL compared to persons with N-NOAB.
Keywords: neurogenic bladder, epidemiology, overactive bladder
Introduction
Neurologic conditions such as stroke, multiple sclerosis, Parkinson’s disease, and spinal cord injury commonly have urologic sequelae in the form of functional disturbances.(1) Disruption to normal urinary function among those with neurologic impairment may be the result of detrusor dysfunction, sphincter dysfunction, or both, and may depend on the site of the neurologic lesion.(2) While nervous system dysfunction is widely recognized as a potential cause of overactive bladder, there is no current research definition based on symptoms for what is variously termed ‘neurogenic bladder’, ‘neurogenic bladder dysfunction’, ‘neurogenic detrusor overactivity syndrome’,(3) ‘neurogenic lower urinary tract dysfunction’ (NLUTD)(1) or ‘neurogenic overactive bladder’.(4) As such, the specific characteristics of persons with neurologic bladder disorders remain poorly understood. New publications based on a recent evidence review and expert panel discussion draw attention to the lack of data estimating the prevalence of NLUTD in the community.(1) (5) To provide specific epidemiologic information to fill this gap, we analyzed data from the Boston Area Community Health (BACH) Survey. Our main objective was to describe and compare the demographic, comorbid, symptom interference and care-seeking characteristics of persons with certain neurologic diseases (ND) who had symptoms of overactive bladder (OAB) to those with OAB symptoms but without a history of ND. We additionally compared the characteristics of those with ND who did and did not have OAB symptoms.
Materials and Methods
Study design and data collection
The BACH Survey is a population-based epidemiologic study conducted among 5,503 persons aged 30-79 years residing in Boston, Massachusetts, USA. A multistage, stratified cluster sampling design was used to recruit approximately equal numbers of participants in pre-specified groups defined by age, race/ethnicity (black, Hispanic, white) and gender. The present analysis used cross-sectional data collected 2002-2005 during a two-hour, in-person interview conducted by bilingual interviewers after written informed consent. Interviews for 63.3% of eligible persons were completed, with a resulting study population of 2301 men and 3202 women (1767 black, 1877 Hispanic, 1859 white). All protocols and procedures were approved by the Institutional Review Board of New England Research Institutes, Inc. Further details of the study methodology are available.(6)
Operational definitions of NOAB and N-NOAB
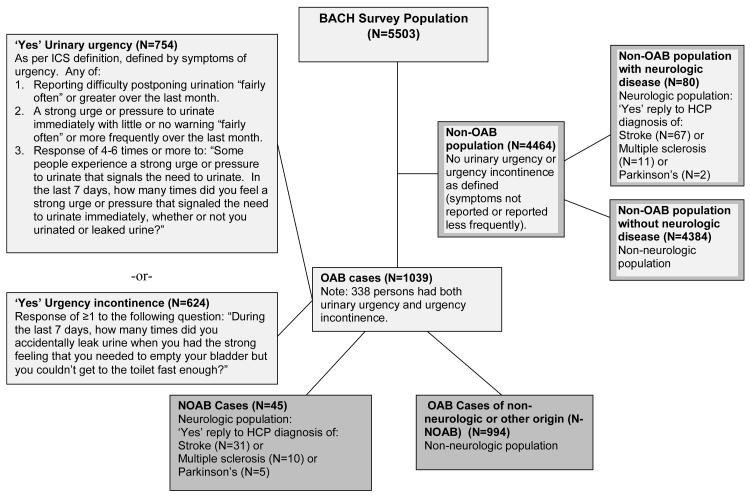
In accordance with the International Continence Society guideline, urgency was treated as the cardinal symptom of overactive bladder (OAB), i.e., having urinary urgency or urgency incontinence (Figure 1).(7) Urinary urgency was defined in this analysis as any of the following: a) reporting difficulty postponing urination “fairly often” or greater over the last month; b) reporting a strong urge or pressure to urinate immediately with little or no warning “fairly often” or more frequently over the last month; or c) a response of 4-6 times or more to: “Some people experience a strong urge or pressure to urinate that signals the need to urinate. In the last 7 days, how many times did you feel a strong urge or pressure that signaled the need to urinate immediately, whether or not you urinated or leaked urine?” Urgency incontinence was defined as a response of ≥1 to the following question: “During the last 7 days, how many times did you accidentally leak urine when you had the strong feeling that you needed to empty your bladder but you couldn’t get to the toilet fast enough?” OAB of presumed neurologic origin (NOAB) was further defined as the presence of symptoms of urgency and/or urgency incontinence (as defined above) among those with a reported history of health care provider (HCP) diagnosed stroke, Parkinson’s disease, or multiple sclerosis. OAB of presumed non-neurologic origin (N-NOAB) was defined using identical symptoms, occurring among those who did not report these conditions.
Figure 1.
Definition and derivation of NOAB and N-NOAB cases from the BACH Survey, 2002-2005.
Other urologic symptoms were measured using the American Urologic Association Symptom Index (AUA-SI) that measures seven urologic symptoms, including incomplete emptying, frequency, intermittency, urgency, weak stream, straining, and nocturia.(8) The AUA-SI was interviewer-administered, and was chosen for use as it is a widely-used instrument with excellent test-retest reliability and has a validated and reliable Spanish version.(8) (9) The scale was originally developed to measure symptoms due to benign prostatic hyperplasia and is validated among men;(8 10) higher scores indicate more symptoms (range 0-35) but captured symptoms are not prostate-specific (e.g., frequency, nocturia). An investigation into the scale’s psychometric properties indicated that its measured reliability among women (as well as convergent and discriminant validity) was similar to that of men in the study, and to that noted by Barry et al. in early investigations of the scale among men.(11) (8) These results suggest that the AUA-SI may be reliably used to measure female LUTS. The presence of LUTS was defined as an AUA-SI score of ≥8, corresponding to typically-used cutpoints for moderate/severe LUTS.(8) The seven individual symptoms were also examined as dichotomous variables and defined as present if reported as occurring “fairly often”/ “usually”/ “almost always” considering the past month.
Covariates
Socioeconomic status (SES) was constructed as a function of standardized income and education variables for the Northeastern United States.(12) Body mass index (BMI) was calculated from interviewer-measured weight and height. Self-reported health status was measured using the 12-item Short Form Health Survey; participants reporting ‘fair’ or ‘poor’ health (combined) were compared with those rating their health ‘good’/’very good’/’excellent’ (combined).(13) Comorbidity variables (high blood pressure, diabetes and arthritis) were based on replies to the query, “Have you ever been told by a health care provider that you have or had …?” while cardiac disease was any report of myocardial infarction and/or angina and/or congestive heart failure and/or coronary artery bypass or angioplasty. Depressive symptoms were considered present among participants reporting at least five of eight symptoms on the abridged Center for Epidemiologic Studies Depression Scale.(14) Physical activity was defined using the Physical Activity Scale for the Elderly (PASE) score (low 0-99, medium 100-249, high 250+).(15)
Symptom interference with activities was assessed using a scale developed by Epstein.(16) A score of 0 was assigned for a reply of “none of the time” while a score of four was assigned to “all of the time” for seven questions on activities (resulting possible score range, 0-28). Activities in the scale are: drinking fluids before travel, drinking fluids before bed, driving, getting enough sleep, going to places without a toilet, playing sports outdoors, and going to movies, shows, etc. The impact of urinary incontinence (UI) on health-related quality-of-life (HRQOL) was measured using a modified version of the Incontinence Impact Questionnaire-7 (score range, 0-21).(17) Variables related to health care access and care-seeking were created from: “How many times in the last year did you go see a health care provider for any reason?” and “Do you go for regular care?” (yes/no). Care-seeking for UI was assessed with, “Have you ever seen a health care provider for your urine leakage?” A “yes” response was followed with, “Did you receive treatment?” and subjects were classified into one of three categories: “No”, “Sought and received treatment” or “Sought but did not receive treatment”.
Participants were asked to gather all medications used in the past four weeks for interviewer recording of label information. In addition, persons were asked if they were taking medications for UI and/or urgency. We combined drugs for UI and overactive bladder due to overlap in use. Subjects taking oxybutynin, tolterodine tartrate, propantheline bromide, and hyoscyamine sulphate were considered to be receiving drug treatment for UI or OAB (newer medications such as darifenacin hydrobromide, solifenacin succinate, and tropsium chloride were not yet available during data collection). Finally, we asked “How important to you would needing to wear a pad or dealing with wetness from leaking urine be for you to seek medical care?” of all participants. Responses indicating inclination to seek care were recorded using a five-item scale, with higher scores indicating greater importance attributed to care-seeking.
Statistical Analysis
Due to the complex sampling design, all analyses were weighted inversely proportional to the probability of being selected, and were conducted using version 10.0.1 of SUDAAN.(18) Missing data were replaced by plausible values using multiple imputation;(19) less than 1% of data were missing for most variables. For income, 3%, 4% and 11% were missing for white, black and Hispanic subjects, respectively. To test differences in stratified analyses, a chi-square test or Fisher’s exact test was performed for categorical covariates and a t-test from linear regression was performed for continuous variables.
Results
Of the 5,503 subjects, 1,039 had symptoms of OAB. Of these, 45 also reported ND, and were classified as having NOAB. The remaining 994 did not have ND and were classified as N-NOAB (Figure 1). Overall, the prevalence of NOAB and N-NOAB in our study population by these definitions was 0.6% and 16.4%, respectively. Considering the 125 persons with ND, the majority reported a history of stroke (98, or 73.0%), followed by MS (21, 23.1%) and Parkinson’s (7, 5.4%), while one person reported both stroke and Parkinson’s. Overall, 45 (31.0%) of the ND group had symptoms of urgency and/or urgency incontinence and met our definition of NOAB. Within subtypes of ND, 80.0% of persons reporting a history of Parkinson’s disease met our definition of NOAB, followed by 50.6% of participants reporting MS, and 22.0% of those reporting a history of stroke (Table I). Persons with ND and NOAB had on average shorter duration of ND than persons with ND without OAB (stroke: 9.1 vs. 11.5 years; MS: 4.6 vs. 15.9 years; Parkinson’s: 2.7 vs. 8.5 years).
Table I.
Prevalence of NOAB among each neurologic disease category, stratified by gender (N=125).
| Men N=57 |
Women N=68 |
Total N=125 |
|
|---|---|---|---|
|
| |||
| N (%) with NOAB |
N (%) with NOAB |
N (%) with NOAB |
|
| Stroke* (N=98) | 17 (21.1) | 14 (23.2) | 31 (22.0) |
| Multiple sclerosis (N=21) | 2 (38.0) | 8 (55.0) | 10 (50.6) |
| Parkinson’s disease (N=7) | 2 (87.2) | 3 (76.3) | 5 (80.0) |
Table notes: Percents shown are row percents. Prevalence estimates are weighted for sampling design. Abbreviations: SE, standard error
One subject had both stroke and Parkinson’s disease. A total of 45 unique subjects had NOAB.
Table II compares demographic, medical characteristics and health behaviors of the groups NOAB, N-NOAB, ND without OAB, and the remaining BACH population without ND or OAB. Comparing NOAB to N-NOAB, there was a slight preponderance of minority race among persons with NOAB, and more persons with NOAB had low SES. There was a higher proportion of persons with obese BMI (59.0%) in the NOAB group compared to the N-NOAB group (44.4%). As would be expected in a population with neurologic conditions such as stroke, there was a higher prevalence of high blood pressure, diabetes, and cardiac disease among the NOAB group compared to the N-NOAB group (all p<0.05). Similarly, persons with NOAB were more likely report fair or poor health (p=0.04), but were not more likely than those with N-NOAB to have depressive symptoms (p=0.78). 68.6% of the NOAB group met the definition of moderate-to-severe LUTS compared to 58.7% of the N-NOAB group.
Table II.
Demographic and medical characteristics of those with NOAB (N=45) vs. those with ND and no OAB (N=80) vs. those with N-NOAB* (N=994) vs. remaining BACH population (N=4384).
| NOAB N=45 |
N-NOAB N=994 |
ND & no OAB N=80 |
Remaining population N=4384 |
|
|---|---|---|---|---|
| Mean age (SE) | 57.4 (3.0) | 53.6 (0.7) | 62.0 (2.0) | 47.1 (0.4) |
|
| ||||
| N (%) | N (%) | N (%) | N (%) | |
|
| ||||
| Race/ethnicity | ||||
| Black | 21 (50.5) | 371 (34.0) | 28 (29.2) | 1347 (26.1) |
| Hispanic | 10 (4.0) | 259 (11.0) | 25 (8.9) | 1583 (13.8) |
| White | 14 (45.5) | 364 (55.0) | 27 (61.8) | 1454 (60.1) |
| Health insurance† | ||||
| Private | 11 (29.4) | 411 (52.1) | 32 (51.7) | 2131 (67.0) |
| Public only | 30 (59.9) | 466 (38.1) | 44 (45.1) | 1463 (20.5) |
| None | 4 (10.7) | 117 (9.8) | 4 (3.2) | 790 (12.4) |
| LUTS‡ | 33 (68.6) | 630 (58.7) | 13 (17.9) | 444 (10.3) |
| Socioeconomic status | ||||
| Lower | 28 (63.4) | 490 (34.4) | 49 (48.2) | 1998 (25.8) |
| Middle | 15 (30.1) | 386 (45.0) | 23 (34.4) | 1729 (47.8) |
| Upper | 2 (6.5) | 118 (20.6) | 8 (17.4) | 656 (26.4) |
| Body mass index (kg/m2) |
||||
| Normal (<25.0) | 9 (23.5) | 189 (27.2) | 21 (36.2) | 1130 (30.7) |
| Overweight (25.0-29.9) | 10 (17.5) | 270 (28.4) | 21 (28.9) | 1576 (35.8) |
| Obese (30.0+) | 26 (59.0) | 535 (44.4) | 38 (34.9) | 1678 (33.6) |
| Self-reported health | 26 (52.6) | 399 (27.2) | 41 (32.8) | 1057 (13.7) |
| (% Poor/Fair)† | ||||
| High blood pressure (% yes)† |
33 (64.2) | 446 (39.8) | 52 (66.0) | 1329 (23.8) |
| Depression (% yes) | 17 (29.2) | 362 (31.6) | 20 (12.3) | 822 (14.3) |
| Diabetes (% yes)† | 18 (35.9) | 210 (15.4) | 21 (21.2) | 498 (7.9) |
| Cardiac disease (% yes) † |
19 (39.6) | 165 (14.7) | 27 (37.7) | 340 (7.1) |
| Arthritis (% yes) | 21 (36.8) | 456 (42.5) | 34 (40.1) | 995 (19.2) |
| Current smoking (% yes) |
20 (34.6) | 297 (31.4) | 19 (25.5) | 1150 (26.6) |
| Physical activity (PASE)† |
||||
| Low | 31 (64.0) | 431 (36.1) | 51 (59.4) | 1372 (24.7) |
| Medium | 11 (34.0) | 445 (49.8) | 23 (25.9) | 2167 (51.4) |
| High | 3 (2.0) | 118 (14.1) | 6 (14.7) | 845 (23.9) |
Table notes: Percents shown are column percents. Means & prevalence estimates are weighted for sampling design.
Abbreviations: NOAB= OAB of presumed neurologic origin, N-NOAB=non-neurogenic OAB, ND=neurologic disease, BACH=Boston Area Community Health Survey, SE= standard error.
This was defined identically to NOAB, except that the requirement to have neurologic disease was removed (see Figure 1).
p<0.05 (NOAB vs. N-NOAB): Chi Square or Fisher’s Exact for categorical variables, t-test for continuous variables
p<0.05 (NOAB vs. ND and no NOAB): Chi Square or Fisher’s Exact for categorical variables, t-test for continuous variables
Comparing the ND population, those with OAB symptoms (NOAB) were of younger mean age (57.4y) compared to those without OAB symptoms (62.0y), but were older than the population with OAB who did not have ND (N-NOAB), where the mean age was 53.6y. There was a higher prevalence of obesity among persons with OAB symptoms compared to those with ND without OAB (59.0% vs. 34.9%, respectively, p=0.15). Except for depression and diabetes, the prevalence of comorbidities were roughly equivalent in the two groups, although the proportion self-reporting fair/ poor health was higher in the NOAB group (52.6% vs. 32.8%, p=0.10).
We examined seven individual symptoms contained in the AUA-SI by OAB and ND status (Table III). Comparing NOAB to N-NOAB, the prevalence of all symptoms was higher among those with NOAB; differences were substantial for voiding-type symptoms such as weak urinary stream. Table IV compares AUA symptom score, symptom interference, use of medical care, and care-seeking for urologic symptoms among the four groups. The mean AUA score was higher among the NOAB group compared to the N-NOAB group (13.0 vs. 10.0, p=0.09). Among persons with OAB, those with NOAB had higher (worse) mean symptom interference scores compared to persons with N-NOAB (9.8 and 5.9, respectively, p=0.03), suggesting that they had more symptom bother. Similarly, UI HRQOL scores were worse among persons with NOAB compared to N-NOAB (6.3 vs. 2.8, p=0.01). The mean number of annual HCP visits in the NOAB group was significantly higher compared to the N-NOAB group (28.8, and 13.7, respectively p=0.009). However, persons in the ND and no OAB group reported 22.8 annual visits to HCPs on average. Among those with urine leakage who were asked a question regarding care-seeking, the NOAB group was also more likely to seek and receive treatment for urine leakage compared to persons with N-NOAB. Correspondingly, the NOAB group had the highest use of relevant medications for urologic conditions (14.6%). However, with respect to ratings of importance to seek care for urine leakage (asked of all participants), the proportion giving a rating of ‘important or extremely important’ to seek care was approximately equal in the two OAB groups
Table III.
Prevalence of other urologic symptoms among those with NOAB (N=45) vs. those with ND and no OAB (N=80) vs. those with N-NOAB* (N=994) vs. remaining BACH population (N=4384).
| NOAB N=45 |
N-NOAB N=994 |
ND & no OAB N=80 |
Remaining population N=4384 |
|
|---|---|---|---|---|
|
| ||||
| N (%) | N (%) | N (%) | N (%) | |
| Incomplete emptying | 13 (28.1) | 234 (18.5) | 3 (2.4) | 119 (2.9) |
| Repeat urination in 2 hrs | 25 (55.1) | 518 (51.6) | 7 (13.0) | 541 (15.4) |
| Difficulty stopping or starting urination |
11 (33.4) | 181 (14.2) | 1 (0.1) | 110 (2.7) |
| Difficulty postponing urination |
22 (47.1) | 438 (43.7) | 0 (0.0) | 0 (0.0) |
| Weak stream† | 14 (34.8) | 163 (12.4) | 3 (4.0) | 97 (2.3) |
| Pushing/straining to begin urination |
8 (16.1) | 83 (8.5) | 2 (2.8) | 47 (1.4) |
| Getting up more than once to urinate at night |
26 (45.3) | 410 (36.9) | 11 (9.4) | 426 (9.3) |
Table notes: Percents shown are column percents. Prevalence estimates are weighted for sampling design. Abbreviations: NOAB= OAB of presumed neurologic origin, N-NOAB=non-neurogenic OAB, ND=neurologic disease, BACH=Boston Area Community Health Survey.
This was defined identically to NOAB, except that the requirement to have neurologic disease was removed (see Figure 1).
p<0.05 (NOAB vs. N-NOAB): Chi Square or Fisher’s Exact
Table IV.
Symptom interference and care-seeking of those with NOAB (N=45) vs. those with ND and no OAB (N=80) vs. those with N-NOAB (N=994) vs. remaining BACH population (N=4384).
| NOAB N=45 |
N-NOAB N=994 |
ND & no OAB N=80 |
Remaining population N=4384 |
|
|---|---|---|---|---|
| Mean AUA score (SE) | 13.0 (1.8) | 10.0 (0.3) | 3.7 (0.6) | 3.1 (0.1) |
| Mean symptom interference score (SE)*,** |
9.8 (1.8) | 5.9 (0.3) | 1.1 (0.3) | 0.9 (0.1) |
| Mean urinary incontinence health- related quality-of-life score (SE)† ** |
6.3 (1.4) | 2.8 (0.2) | 0.0 (0.0) | 0.1 (0.0) |
| Mean annual frequency of visits to any HCP (median)** |
28.8 (15.0) | 13.7 (6.0) | 22.8 (10.0) | 8.1 (4.0) |
|
| ||||
| N (%) | N (%) | N (%) | N (%) | |
|
| ||||
| % Reporting visits for regular care** |
45 (100.0) | 913 (91.6) | 79 (97.9) | 3698 (84.3) |
| Have you seen HCP for urine leakage? ‡ ** |
||||
| No | 20 (52.8) | 446 (67.2) | 7 (100.0) | 388 (84.4) |
| Sought and received treatment | 9 (43.3) | 130 (16.1) | 0 (0.0) | 56 (8.9) |
| Sought but did not receive treatment |
5 (3.8) | 142 (16.8) | 0 (0.0) | 46 (6.6) |
| % Receiving urologic drug treatment§ |
4 (14.6) | 41 (3.7) | 2 (4.2) | 22 (0.5) |
| % Rating important or extremely important to care-seek for urine leakage¶ |
40 (87.0) | 885 (88.8) | 76 (97.2) | 4055 (92.8) |
Table notes: Percents shown are column percents. Means & prevalence estimates are weighted for sampling design.
Abbreviations: NOAB=OAB of presumed neurologic origin, N-NOAB=non-neurogenic OAB, ND=neurologic disease, BACH=Boston Area Community Health Survey, SE=standard error.
Based on the Epstein scale for symptom interference with activities (higher=worse interference, possible range 0-28).
Based on a modified version of the Incontinence Impact Questionnaire Short Form (IIQ-7) (higher score=worse impact, possible range 0-21).
Survey question asked only among those who reported incontinence, hence, observed N for this variable does not equal column total.
Included UI/OAB meds are oxybutynin, tolterodine tartrate, propantheline bromide, and hyoscyamine sulphate. Newer medications such as darifenacin hydrobromide, solifenacin succinate, and tropsium chloride were not yet available at the time of our data collection.
Replies to query, “How important to you would needing to wear a pad or dealing with wetness from leaking urine be for you to seek medical care? Would you say..[5 categories ranging from extremely unimportant to extremely important]”.
p<0.05 (NOAB vs. N-NOAB): Chi Square or Fisher’s Exact for categorical variables, t-test for continuous variables
DISCUSSION
Our community-based study may provide the first prevalence estimate of NOAB in a community setting. It is likely that persons with NOAB in our study have less severe symptoms than what may occur in non-community-dwelling patients, e.g. those in long-term care, whose urologic symptoms may be related to their admission. We observed that the majority of persons with ND did not meet our definition of having NOAB. Within the ND group, obesity and diabetes were (non-significantly) associated with having OAB, but older age, cardiac disease, and hypertension (traditional risk factors for LUTS) were not. The lack of association of older age with OAB within the ND group was surprising, as was the reported shorter duration of time since the ND event among those with NOAB. We speculate that these may be explained by development of non-medical coping strategies over time, and/or betterment of underlying ND and subsequent symptoms over time/aging. Regardless, compared to OAB of other origin among community-dwelling men and women, our overall findings suggest that NOAB in our population represented a greater burden of urologic illness, given there was a higher co-prevalence of other urologic symptoms including voiding symptoms, overall AUA scores were (non-significantly) higher, and scores on symptom interference and HRQOL instruments relevant to the impact of urologic symptoms were substantially worse.
Prior studies of specific ND populations such as MS and Parkinson’s have estimated the prevalence of urologic symptoms, although direct comparisons to our work are difficult due to a lack of uniform definition of NOAB and our small subgroup sample size. In population-based studies, the prevalence of urologic symptoms among persons with MS was 44% (with urgency and frequency most common)(20) and 57.5%,(21) similar to our estimate of 50.6% of MS patients with NOAB. The proportion of NOAB among persons with Parkinson’s (80%) is higher than the published prevalence of urgency (36.3%) and urgency-related incontinence (20.9%) from one small clinical study,(22) but because our proportion is based on very small numbers (n=7) these results should be interpreted with caution.
A recently-published analysis of neurogenic bladder patients based on a large dataset of medical claims provides some comparisons for our results with respect to demographics, underlying neurologic disease, and use of medical care.(23) Persons who had relevant claim codes for neurogenic bladder, OAB plus a neurologic disorder, or use of an OAB drug plus a neurologic disorder were selected for inclusion into a cohort of 46,271 neurogenic bladder patients. The cohort mean age was 62.5 with a mean of 16.1 visits to medical care in the year following qualifying diagnoses (similar to our median of 15.0 visits annually). The proportion of patients who were continuing users of medications for OAB was much higher in this study compared to current users in BACH (29% vs. 14.6%, respectively). This is likely due to medication use as a cohort inclusion criteria, as well as the insurance-based study setting. While the study did not examine specific urologic symptoms, a high co-prevalence of other urologic comorbidities such as lower urinary tract infections, urinary retention and obstructive uropathies suggest (similar to our study) that those with NOAB are highly symptomatic.
We found that persons with NOAB were more likely than those with N-NOAB to seek care and receive treatment for UI. We also observed that they were substantially more bothered by their symptoms. In a past analysis of this population, we documented that symptom bother was associated with receiving drug treatment for a variety of urologic symptoms.(24) The present findings may also reflect a greater willingness on the part of providers to treat OAB related to ND, which may be viewed as less transitory.
Our study has limitations in that the number of persons who met our NOAB definition was small and restricted our ability to examine any differences by ND subtypes. Despite the heterogeneity of underlying neurologic disease in our study, urodynamic evaluations most often reveal similar findings in these groups (typically detrusor overactivity), suggesting our grouping them is not inappropriate.(25 26) In an epidemiologic study that is representative of and generalizable to the underlying source population aged 30-79 such as the BACH Survey, a small number of persons with a history of ND is to be expected. Reflecting the rank of stroke as the neurologic condition of highest incidence among U.S. adults,(27) most (73%) of our neurologic population was comprised of stroke patients. Not all NDs were collected in the survey, however, and some misclassification is likely. An additional limitation is that the NOAB definition was partially based on self-report of ND, and urologic symptoms were presumed to be of neurologic origin. Self-report of stroke history has been found to have an accuracy or positive predictive value ranging from 0.67 to 0.79 (28) (29) (30) when compared to medical records or clinical evaluation. Our analysis was cross-sectional; cause and effect in the observed associations cannot be assumed. Our NOAB and N-NOAB definitions were based on reported symptoms. While the specific clinical features of these symptoms among persons in our study are unknown, symptom-based studies provide a more complete understanding of the burden of urologic conditions in the general population, especially in a urban U.S. setting where many lack health insurance, allowing us to consider persons who do not present to care. Further strengths of the study include the diversity of race, ethnicity, and gender. The generalizability of the BACH population is known. Medicare coverage and health insurance coverage in BACH were similar to other major U.S. health surveys (National Health and Nutrition Examination Survey, National Health Interview Survey and Behavioral Risk Factor Surveillance Survey), as were the distributions of common comorbidities (except asthma, more common in BACH).(6)
Conclusions
Our analysis may have clinical implications in that persons with a history of ND with OAB may require closer follow-up and more frequent intervention for bothersome urologic symptoms than those with OAB of other etiology. Future studies might focus on differences in treatment efficacy in neurogenic versus non-neurogenic populations, given the findings noted in this large cohort.
Acknowledgments
Funding source and role of the funding source:
Funding for the BACH Survey was provided by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (NIH) DK 56842. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. Additional support for these analyses was provide to New England Research Institutes, Inc., from Allergan, Inc. The NIDDK funding source had no role in the study design; in the collection, analysis or interpretation of data or in the decision to submit the manuscript for publication. The Allergan funding source had no role in the study design; in the collection, analysis of data or in the decision to submit the paper for publication. An Allergan author contributed to the interpretation of analyzed data and to the writing of this manuscript.
REFERENCES
- 1.Stohrer M, Blok B, Castro-Diaz D, Chartier-Kastler E, Del Popolo G. EAU guidelines on neurogenic lower urinary tract dysfunction. Eur Urol. 2009;56(1):81–88. doi: 10.1016/j.eururo.2009.04.028. others. [DOI] [PubMed] [Google Scholar]
- 2.Wyndaele JJ, Kovindha A, Madersbacher H, Radziszewski P, Ruffion A. Neurologic urinary incontinence. Neurourol Urodyn. 2010;29(1):159–164. doi: 10.1002/nau.20852. others. [DOI] [PubMed] [Google Scholar]
- 3.Chancellor MB, Anderson RU, Boone TB. Pharmacotherapy for neurogenic detrusor overactivity. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2006;85(6):536–545. doi: 10.1097/01.phm.0000219229.92056.c2. [DOI] [PubMed] [Google Scholar]
- 4.Fall M, Ohlsson BL, Carlsson CA. The neurogenic overactive bladder. Classification based on urodynamics. Br J Urol. 1989;64(4):368–373. doi: 10.1111/j.1464-410x.1989.tb06045.x. [DOI] [PubMed] [Google Scholar]
- 5.Wyndaele JJ, Bruschini H, Madersbacher H, Moore K, Pontari M. Neurological patients need evidence-based urological care. Neurourol Urodyn. 2010;29(4):662–669. doi: 10.1002/nau.20866. others. [DOI] [PubMed] [Google Scholar]
- 6.McKinlay JB, Link CL. Measuring the Urologic Iceberg: Design and Implementation of the Boston Area Community Health (BACH) Survey. Eur Urol. 2007;52(2):389–396. doi: 10.1016/j.eururo.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167–178. doi: 10.1002/nau.10052. others. [DOI] [PubMed] [Google Scholar]
- 8.Barry MJ, Fowler FJ, Jr., O’Leary MP, Bruskewitz RC, Holtgrewe HL. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549–1557. doi: 10.1016/s0022-5347(17)36966-5. others. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 9.Badia X, Garcia-Losa M, Dal-Re R, Carballido J, Serra M. Validation of a harmonized Spanish version of the IPSS: evidence of equivalence with the original American scale. International Prostate Symptom Score. Urology. 1998;52(4):614–620. doi: 10.1016/s0090-4295(98)00204-0. [DOI] [PubMed] [Google Scholar]
- 10.Barry MJ, Fowler FJ, Jr., O’Leary MP, Bruskewitz RC, Holtgrewe HL. Correlation of the American Urological Association symptom index with self-administered versions of the Madsen-Iversen, Boyarsky and Maine Medical Assessment Program symptom indexes. Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1558–1563. doi: 10.1016/s0022-5347(17)36967-7. others. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 11.Okamura K, Nojiri Y, Osuga Y, Tange C. Psychometric analysis of international prostate symptom score for female lower urinary tract symptoms. Urology. 2009;73(6):1199–1202. doi: 10.1016/j.urology.2009.01.054. [DOI] [PubMed] [Google Scholar]
- 12.Green LW. Manual for scoring socioeconomic status for research on health behavior. Public Health Rep. 1970;85(9):815–827. [PMC free article] [PubMed] [Google Scholar]
- 13.Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr. 1999;11(2):139–148. doi: 10.1017/s1041610299005694. [DOI] [PubMed] [Google Scholar]
- 15.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 16.Epstein RS, Deverka PA, Chute CG, Panser L, Oesterling JE. Validation of a new quality of life questionnaire for benign prostatic hyperplasia. J Clin Epidemiol. 1992;45(12):1431–1445. doi: 10.1016/0895-4356(92)90205-2. others. [DOI] [PubMed] [Google Scholar]
- 17.Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA. Short forms to assess life quality and symptom distress for urinary incontinence in women: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women Research Group. Neurourol Urodyn. 1995;14(2):131–139. doi: 10.1002/nau.1930140206. [DOI] [PubMed] [Google Scholar]
- 18.Research Triangle Institute . SUDAAN Language Manual Release 9.0. Research Triangle Park; NC: 2004. [Google Scholar]
- 19.Schafer JL. Analysis of incomplete multivariate data. Chapman & Hall; New York: 1997. p. xiv.p. 430. [Google Scholar]
- 20.Hennessey A, Robertson NP, Swingler R, Compston DA. Urinary, faecal and sexual dysfunction in patients with multiple sclerosis. Journal of neurology. 1999;246(11):1027–1032. doi: 10.1007/s004150050508. [DOI] [PubMed] [Google Scholar]
- 21.Bakke A, Myhr KM, Gronning M, Nyland H. Bladder, bowel and sexual dysfunction in patients with multiple sclerosis--a cohort study. Scandinavian journal of urology and nephrology. 1996;179:61–66. [PubMed] [Google Scholar]
- 22.Sammour ZM, Gomes CM, Barbosa ER, Lopes RI, Sallem FS. Voiding dysfunction in patients with Parkinson’s disease: impact of neurological impairment and clinical parameters. Neurourol Urodyn. 2009;28(6):510–515. doi: 10.1002/nau.20681. others. [DOI] [PubMed] [Google Scholar]
- 23.Manack A, Motsko SP, Haag-Molkenteller C, Dmochowski RR, Goehring EL., Jr. Epidemiology and healthcare utilization of neurogenic bladder patients in a us claims database. Neurourol Urodyn. 2010 doi: 10.1002/nau.21003. others. [DOI] [PubMed] [Google Scholar]
- 24.Hall SA, Link CL, Hu JC, Eggers PW, McKinlay JB. Drug treatment of urological symptoms: estimating the magnitude of unmet need in a community-based sample. BJU Int. 2009 doi: 10.1111/j.1464-410X.2009.08686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray S, Lemack GE. Multiple Sclerosis and Voiding Dysfunction in Women. Curr Bladder Dysfunct Rep. 2011 (in press) [Google Scholar]
- 26.Nitti VW, Adler H, Combs AJ. The role of urodynamics in the evaluation of voiding dysfunction in men after cerebrovascular accident. J Urol. 1996;155(1):263–266. [PubMed] [Google Scholar]
- 27.Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR. How common are the “common” neurologic disorders? Neurology. 2007;68(5):326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. others. [DOI] [PubMed] [Google Scholar]
- 28.Bergmann MM, Byers T, Freedman DS, Mokdad A. Validity of self-reported diagnoses leading to hospitalization: a comparison of self-reports with hospital records in a prospective study of American adults. Am J Epidemiol. 1998;147(10):969–977. doi: 10.1093/oxfordjournals.aje.a009387. [DOI] [PubMed] [Google Scholar]
- 29.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57(10):1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Engstad T, Bonaa KH, Viitanen M. Validity of self-reported stroke : The Tromso Study. Stroke. 2000;31(7):1602–1607. doi: 10.1161/01.str.31.7.1602. [DOI] [PubMed] [Google Scholar]