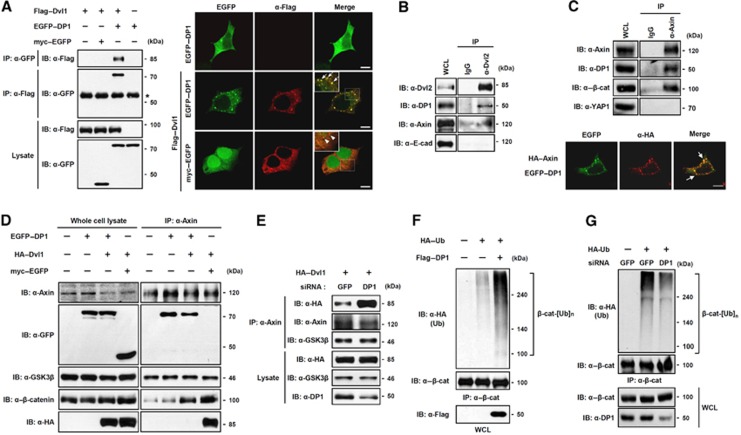
Figure 2.
DP1 inhibits Dvl–Axin interaction and enhances poly-ubiquitination of β-catenin. (A) DP1 associated with Dvl. Cells were transfected with plasmids as indicated and subjected to immunoprecipitation (IP) and immunofluorescence (IF). Asterisk indicates IgG heavy chain. Arrows indicate co-localized puncta between EGFP–DP1 and Flag–Dvl1. Scale bar, 10 μm. (B) Interaction between endogenous DP1 and Dvl. (C) IP was performed to show the interaction between endogenous DP1 and Axin (top). IF was performed in cells transfected with EGFP–DP1 and HA–Axin. Arrows indicate co-localized puncta (bottom). Scale bar, 10 μm. (D) HA–Dvl1 was co-transfected with either myc–EGFP or EGFP–DP1. IP was performed with anti-Axin antibody. (E) Cells expressing siGFP or siDP1 were transfected with HA–Dvl1. IP was performed with anti-Axin antibody. (F, G) HA–Ub was transfected with or without Flag–DP1 (F). HA–Ub was transfected with siGFP or siDP1 RNA (G). Cell lysates were immunoprecipitated with anti-β-catenin antibody and analysed by Western blotting using antibodies indicated.