Abstract
Objective
The purpose of this study was to present the characteristics and outcome of patients with proven pheochromocytoma or paraganglioma who had false-negative 123I-MIBG SPECT.
Methods
Twenty one patients with false-negative 123I-MIBG SPECT, (6 males, 15 females) aged 13–55 years (mean 40.9 years) were included. We classified them according to the stage of the disease as non-metastatic or metastatic at the time of false-negative 123I-MIBG SPECT study, the location and size of the tumor, plasma and urinary catecholamine and metanephrine levels, genetic mutations, and outcome in terms of occurrence and progression of metastases and death.
Results
Thirteen patients were evaluated for metastatic tumors while 8 others were seen for non-metastatic disease. All primary tumors and multiple metastatic foci did not show avid 123I-MIBG uptake regardless of the tumor diameter. The majority of patients had extra-adrenal tumors with hypersecretion of normetanephrine or norepinephrine. SDHB mutation was present in 52% (n=11) of cases, RET mutation in 4% (n=1), and the rest were apparently sporadic. Twenty four percent (n=5) had metastatic disease on initial presentation. Fourteen patients were followed-up for 3–7 years. From them, 71% (n=10) had metastatic disease and majority had SDHB mutation. Nine are still alive while 5 (4 were SDHB) died due to metastatic disease.
Conclusion
A false-negative 123I-MIBG SPECT is frequently related to metastatic tumors and usually due to SDHB mutations with unfavourable prognosis. We, therefore, recommend that patients with false-negative 123I-MIBG SPECT be tested for SDHB mutations and to undergo more regular and close follow-up.
Keywords: 123I-MIBG, pheochromocytoma, paraganglioma, succinate dehydrogenase subunit B, catecholamines, metanephrines
INTRODUCTION
Pheochromocytoma (PHEO) and paragangliomas (PGL) are tumors arising from chromaffin cells of the adrenal medulla or extra-adrenal paraganglionic tissues, respectively (DeLellis et al. 2004). These tumors express cellular membrane norepinephrine transporter (NET) through which catecholamines can enter and deposited inside neurosecretory granules via the vesicular monoamine transporter systems (VMATs). Metaiodobenzylguanidine (MIBG) is a guanethidine analogue resembling norepinephrine (NE) that can enter chromaffin cells through active uptake via NET or passive diffusion and is stored in catecholamine containing neurosecretory granules (Vaidyanathan 2008, Havekes et al. 2008, Bomanji et al. 1987, Sisson et al. 1981). This characteristic make MIBG very useful and extremely specific for the diagnostic localization of PHEO and PGL when labelled with radiotracers such as iodine-123 (123I) or iodine-131 (131I) (Jacobson et al. 2010, Meyer-Rochow et al. 2010, Havekes et al. 2008, Ilias and Pacak 2004, Timmers et al. 2009, Sisson et al. 1981, Goldsmith 2009). In addition, 131I-MIBG is also used for the treatment of metastatic PHEO and PGL that demonstrate avid MIBG uptake (Loh et al. 1997, Gonias et al. 2009, Rose et al. 2003, Castellani et al. 2010).
It was previously shown that 123/131I-MIBG scintigraphy is comparable with other nuclear imaging studies such as 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography (18F-FDG PET), [18F]-fluorodopamine (18F-FDA)and [18F]-fluorodopa (18F-DOPA) in the detection of primary, sporadic, and non-metastatic PHEO and PGL (Timmers et al. 2009). However, the sensitivity of 123/131I-MIBG scintigraphy in the detection of metastatic tumors and familial PHEO and PGL is somewhat suboptimal compared with other nuclear imaging studies (van der Harst et al. 2001, Taïeb et al. 2004, Ilias et al. 2003, Fottner et al.2010, Kauhanen et al. 2009, Fiebrich et al. 2009, Goldsmith 2009, Shulkin et al. 1999). It is also less sensitive in the detection of extra-adrenal and small PHEO and PGL (van der Harst et al. 2001, Bhatia et al. 2008, Wiseman et al. 2009). Since the uptake of MIBG was shown to be well correlated with the presence of NET or VMATs; the number of neurosecretory granules and perhaps the degree of differentiation of tumor cells controlling the expression of NET may play an important role in a successful application of 123/131I-MIBG scintigraphy (Bomanji et al. 1987, Eisenhofer et al. 2001, Fottner et al. 2010). Nevertheless, 123/131I-MIBG scintigraphy remains widely used because it is readily available and less expensive than 18F-FDA and 18F-FDOPA PET.
Initially we observed that patients with PHEO and PGL, especially those growing rapidly, presented with negative 123I-MIBG single photon emission computed tomography (SPECT). Then some of them with rapidly progressing PHEO and PGL were found to be associated with succinate dehydrogenase subunit B (SDHB) gene mutation. Therefore, we hypothesized that negative 123I-MIBG SPECT, regardless whether in patients having a primary or recurrent tumor or metastatic disease, would point towards the presence of SDHB mutation. Proving this hypothesis would alert physicians to initiate SDHB mutation testing, especially in those patients with a negative family history of this disease. Furthermore, we hypothesized that these 123I-MIBG SPECT negative tumors could reflect more aggressive behavior (as also commonly seen in SDHB patients) and should also alert physicians to perform more regular follow-up including biochemical as well as imaging tests.
MATERIALS AND METHODS
Patients
Official results of 123I-MIBG SPECT of patients seen at the National Institutes of Health (NIH) from 2002 through March 2011 for evaluation of PHEO and PGL were reviewed. Patients with false-negative 123I-MIBG SPECT at any point from initial presentation to follow-up were identified and included in the present study if they were diagnosed with PHEO and PGL based on the clinical presentation, specific biochemical tests including measurement of catecholamine and metanephrines in either plasma or urine, PHEO and PGL specific imaging studies, and histopathological confirmation of resected tumors. All patients were part of an Institutional Review Board approved prospective study of patients with known or suspected PHEO and/or PGL at NIH. All patients provided informed consent.
Biochemical Tests
Patients were asked to abstain from acetaminophen for 5 days, caffeinated and decaffeinated products, smoking, and alcohol for 24 hours prior to blood extraction and 24-hoururine collection. For plasma catecholamine and metanephrine determination, a cannula was inserted in the forearm for intravenous access. Patients were in the supine position without a pillow in a quiet room for 20–30 minutes before and during collection. As soon as blood was collected, it was placed on ice and stored in −80°C until testing. Basal plasma levels of catecholamines and metanephrines were measured by high performance liquid chromatography (HPLC). For urinary catecholamine and metanephrine determination, total volume collected over 24 hours was used and measured by HPLC or liquid chromatography-tandem mass spectrometry.
Imaging Tests
Computed Tomography (CT)
Axial images of the neck, chest, abdomen, and pelvis were obtained after administration of oral and intravenous low-osmolar contrast. Multiple helical axial images at 2.5 mm and 5 mm thick were obtained in the neck and from the thoracic inlet to the symphysis pubis, respectively.
Magnetic Resonance Imaging (MRI)
Axial images of the head, neck, chest, abdomen and pelvis were obtained. Axial T1, axial STIR, and post-contrast fat-saturated axial T1-weighted images were obtained through the neck while T1- and T2-weighted scans and STIR images were obtained in the chest. Scans were obtained before, during, and after intravenous injection of 14 mL Magnevist. In the abdomen, multiple sequences including axial T2-weighted (one without fat suppression with respiratory trigger; another one with fat suppression and suspended respiration) and 2D in and out of phase T1 weighted images prior to, and multiphase 3D volume images in axial planes, single venous coronal following vascular contrast administration (18cc Magnevist) obtained at 3T. If clinically indicated, MRI of the spine was obtained with sagittal T1-weighted and sagittal STIR images of the cervical, thoracic, and lumbar spine without administration of contrast material. Axial T2-weighted images were acquired at selected levels.
123I-MIBG SPECT
Patients were required to withdraw medications that can potentially interfere with MIBG uptake (e.g. labetalol, monoamine oxidase inhibitors, phenylpropanolamine, tricyclic and other antidepressants, or reserpine) at least 1 week before the procedure. To protect the thyroid from accumulation of free radioactive iodine, patients received 100 mg of saturated solution of potassium iodide by mouth twice a day for 4 days starting the night before 123I-MIBG administration. Whole body planar and single photon emission computed tomography imaging were done mostly 24 hours and some 48 hours after intravenous administration of 8.4–11 mci (mean 10.18) 123I-MIBG. Attenuation CT scan was available in 3 patients.
Other Functional Imaging Studies
18F-FDG PET/CT, 18F-FDA PET/CT, 18F-FDOPA PET were performed. The patients were asked to fast for at least 6 hrs prior to intravenous injection of 18F-FDA (1 mci), 18F-FDG (15.9 mci), and 18F-FDOPA (12 mci). Patients were also asked to refrain from caffeine, tobacco and alcohol for at least 12 hrs. Capillary blood glucose was measured before 18F-FDG PET/CT. A low-dose, noncontrast, nondiagnostic CT was obtained for attenuation correction and anatomic localization. For 18F-FDOPA PET, patients received 2 mg/kg carbidopa orally as pretreatment 1 hr before radiotracer injection.
Genetic Testing
Genetic testing was performed at the Department of Human Genetics of the Pittsburgh University Medical Center Clinic, Pennsylvania and at the Mayo Clinic Laboratories, Minnesota, USA. A stepwise approach to genetic testing was performed based on the most likely gene mutation to be present based on the clinical presentation, biochemical phenotype, and the location of tumor(s). Patients were tested for von Hippel-Lindau, multiple endocrine neoplasia type 2 (MEN 2), SDHB, succinate dehydrogenase subunit C, and D gene mutations (Maher and Eng 2002). Not all currently known susceptibility genes for PHEO and PGL were tested in our patients if a specific gene mutation causing the disease was already found. Furthermore, gene testing for TMEM127, SDHA, SDHAF2, and MAX gene mutations was not performed (Hensen and Bayley 2011, Comino-Méndez et al. 2011).
Data Analysis
123I-MIBG studies were reviewed separately by 2 experienced nuclear medicine physicians (C.C. and J.R.) who were blinded to any anatomic imaging studies and clinical history of patients. Physiologic uptake in the adrenal gland was carefully distinguished from abnormal uptake using the widely accepted four-point visual scale: (0) no increased activity demonstrated in one or both adrenal glands; (1) faint increased activity demonstrated in one or both adrenal glands; (2) moderate increased activity in one or both adrenal glands less than or equal to that of the liver; (3) intense increased activity in one or both adrenal glands greater than the liver. Scores of 0 to 2 were classified negative or physiologic uptake while score of 3 was classified positive for PHEO or PGL.
All patients with adrenal tumor had histopathological confirmation of PHEO. Thus, a false-negative 123I-MIBG scan among these patients was defined as the absence of, or radiotracer uptake in the adrenal gland less than that of the liver (score 0–2). On the other hand, patients with multiple metastatic lesions or inoperable tumors may not have pathologic confirmation of metastatic PHEO or PGL. Therefore, a false-negative 123I-MIBG scan in this group was defined as the absence of radiotracer uptake in a tumor of a patient who had clinical, biochemical, and anatomical and specific nuclear imaging studies clearly consistent with PHEO and PGL.
Metastatic PHEO or PGL was defined as the presence of tumor at sites where chromaffin tissues are not normally located such as bones, liver, lungs, and lymph nodes. Multifocal disease was defined as presence of multiple tumor foci, presenting synchronously or metasynchronously (new primary) to the original tumor, whereas recurrence was defined as reappearance of the disease as documented at reintervention or by combined biochemical and radiological tests after previous complete eradication of the tumor.
RESULTS
Patient Characteristics
We included 21 patients (6 males, 15 females) aged 13–55 years (mean 40.9 years), 7 of whom were referred for evaluation and management of possible PHEO and PGL while 14 other patients were referred after previous surgery of their primary tumor (adrenal PHEO, n=4; urinary bladder PGL, n=3; retroperitoneal and extra-adrenal PGL, n=6; carotid body tumor, n=1) (Table 1). After a thorough evaluation, 8 patients were found to have non-metastatic tumor and 13 others have metastatic disease.
TABLE 1.
Characteristics of Patients at the Time of False Negative 123I-MIBG SPECT
| Patient | Age (yr)/Sex | Reason for consult at NIH | Hypersecretion in Plasma | Hypersecretion in Urine | Gene Mutation | ||
|---|---|---|---|---|---|---|---|
| Meta-nephrines | Cate-cholamines | Meta-nephrines | Cate-cholamines | ||||
| 1 | 13/F | P | NMN, MN | NE, E, DA | -- | -- | Apparently sporadic |
| 2 | 47/F | P | NMN, MN | E | NMN, MN, T | None | RET |
| 3 | 51/F | P | NMN, MN | NE, E | NMN, MN, T | NE, E | Apparently sporadic |
| 4 | 61/M | P | None | DA | -- | -- | SDHB |
| 5 | 49/F | P | NMN | NE | NMN, T | NE | Apparently sporadic |
| 6 | 46/F | P | NMN, MN | None | -- | -- | Apparently sporadic |
| 7 | 36/F | New P | NMN | NE | NMN, T | NE | SDHB |
| 8 | 40/F | R | NMN | NE | NMN, T | NE, DA | Apparently sporadic |
| 9 | 55/M | Met + New P | NMN | NE, DA | NMN, T | NE | Neg for SDHx |
| 10 | 48/F | Met + New P | NMN | NE, DA | NMN, T | NE, DA | SDHB |
| 11 | 42/F | Met + New P | NMN | NE, DA | NMN, T | NE | SDHB |
| 12 | 36/F | Met | NMN | NE | NMN, T | NE | SDHB |
| 13 | 32/M | Met | NMN, MN | None | None | None | Apparently sporadic |
| 14 | 47/F | Met | None | None | -- | -- | SDHB |
| 15 | 43/F | Met | NMN | NMN | NMN, T | None | SDHB |
| 16 | 34/M | Met | NMN | NE, DA | -- | -- | SDHB |
| 17 | 37/M | Met | NMN | NE, DA | -- | -- | SDHB |
| 18 | 43/F | Met | None | None | -- | -- | SDHB |
| 19 | 45/F | Met | NMN | NE | None | NE | Apparently sporadic |
| 20 | 33/M | Met | None | None | -- | -- | SDHB |
| 21 | 22/F | Met | NMN | NE | NMN, T | NE | Apparently sporadic |
Adr: adrenal gland; DA: dopamine; E: epinephrine; F: female; M: male; MN: metanephrine; Met: metastasis; NE: norepinephrine; Neg: Negative; NMN: normetanephrine; P: primary tumor; R: recurrence; RET: rearranged during transfection; SDHB: succinate dehydrogenase subunit B; SDHD: succinate dehydrogenase subunit D; T: total metanephrines; VHL: von Hippel-Lindau
--: not available
Plasma metanephrines were elevated in 17 patients (81%) [5 had both normetanephrine (NMN) and metanephrine (MN) elevated, 12 had elevated NMN only] while 4 others had normal levels. Plasma catecholamines were elevated in 15 patients (71%) [1 had elevated NE, epinephrine (EPI), and dopamine (DA), 1 had elevated NE and EPI, 5 had elevated NE and DA, 6 had elevated NE only, 1 had elevated EPI only, and 1 had elevated DA only]. Urinary metanephrines and catecholamines were available in 13 patients only. Eleven patients (85%) had elevated urinary metanephrines (2 had elevated total metanephrines, NMN and MN; 9 had elevated total metanephrines and NMN). Ten patients (83%) had elevated urinary catecholamines (1 had elevated NE and EPI, 2 had elevated NE and DA, 7 had elevated NE only).
Results of anatomical and functional imaging studies are presented in Table 2. All patients had tumors (primary or metastatic) that failed to accumulate 123I-MIBG at first presentation to NIH except patients no. 11, 15, 18, and 19 in whom false-negative scans were observed on follow-up (second 123I-MIBG SPECT) when they developed a metastatic disease (patient no. 18) or progression of a known metastatic disease (patients no. 11, 15, and 19). Of the 8 patients with non-metastatic tumor (patients no. 1–8), anatomical and nuclear imaging studies other than 123I-MIBG SPECT/CT showed adrenal gland PHEO (n=6; 5 unilateral, 1 bilateral), paraesophageal PGL (n=1), and paraaortic PGL (n=1) which were confirmed by histopathology after surgery. In patient no. 2 with MEN 2 syndrome, 18F-FDG PET/CT further showed increased uptake of radiotracer in the skull, neck, and mediastinum which were proven to be related to medullary tumor of the thyroid after excision. None of them was found to have multifocal and metastatic disease as proven by multiple imaging studies together with normalization of plasma metanephrines and catecholamines postoperatively.
TABLE 2.
Comparative Imaging Studies of Patients with False-Negative 123I-MIBG SPECT
| Patient | CT | MRI | 123I-MIBG | 18F-FDA | 18F-FDOPA | 18F-FDG-PET |
|---|---|---|---|---|---|---|
| 1 | R adr | NA | Neg | NA | NA | R adr |
| 2 | R+L adr | R+L adr | Neg | Neg | NA | R parietal skull*, R upper posterior neck*, R mediastinum* |
| 3 | L adr | L adr | Neg | L adr | NA | L adr |
| 4 | L paraaortic L renal mass** |
L paraaortic | Neg | Neg | Neg | L paraaortic, and L kidney |
| 5 | R adr | R adr | Neg | R adr | NA | NA# |
| 6 | R+L adr | R+L adr | Neg | Neg | R adr | NA |
| 7 | Para-esophageal | Para-esophageal | Normal adr uptake | Neg | NA | Paraesophageal |
| 8 | R adr | NA | Neg | Neg | NA | Neg |
| 9 | Liver, lung, LN, B, bilateral carotid | Liver, LN, B, bilateral carotid | Neg | B | B, Liver, LN | R carotid, soft tissues adjacent to C2 and C3 cervical vertebrae, B, liver, LN |
| 10 | B, LN, lungs, R adr | NA | Normal adr uptake | LN | NA | NA |
| 11 | B, lungs, Liver periaortic | R adr B, liver, retro-peritoneal L periaortic | Normal adr uptake | Liver, bone, LN, soft tissues in the abdomen | NA | B, L upper abdomen in the region of the pancreas and toward the L side of the upper abdomen, liver, LN and soft tissues in the abdomen |
| 12 | Lung, pelvic LN | Lung, pelvic LN | Normal adr uptake | Neg | NA | Lungs, pelvic LN^^^ |
| 13 | B | B | Normal adr uptake | B | NA | B |
| 14 | Lung, LN, B | B^ | Normal adr uptake | Neg | Neg | B, Lungs |
| 15 | B, liver, LN, Infrasplenic | B, liver, LN | Normal adr uptake | B, infrasplenic | NA | R posterior medial lung, B, L lateral abdomen, liver |
| 16 | B, lungs, LN, liver, L paraaortic mass, intrathoracic prevertebral mass | B, liver, LN, L paraaortic mass, intrathoracic prevertebral mass^ | Normal adr uptake | Neg | NA | Lungs, LN, B, liver lower abdomen extending to the midline |
| 17 | LN, lungs, B, liver | B, LN, liver | Normal adr uptake | LN | Neg | LN, B, liver |
| 18 | LN | LN | Normal adr uptake | Neg | NA | B, LN |
| 19 | Lungs, LN | Lungs, LN | Normal adr uptake | R mediastinum at the hilar area, L lung, LN | NA | Lungs, LN |
| 20 | R carotid LN in the chest | R carotid LN in the chest | Neg | Neg | R carotid | LN in the chest |
| 21 | LN | LN | Normal adr uptake | LN | LN | LN |
Adr: adrenal gland, B: bones, L: left, LN: lymph nodes, Neg: Negative; R: right; 18F-FDA:18F-fluorodopamine, 18F-FDOPA:18F-dihydroxyphenylalanine, 18F-FDG PET: 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography
Medullary Thyroid Cancer-related
Left renal mass: renal cell carcinoma by histopathology
available from outside prior to referral to NIH with increased uptake in the right adrenal gland
MRI of the chest not done
outside scan not available for review
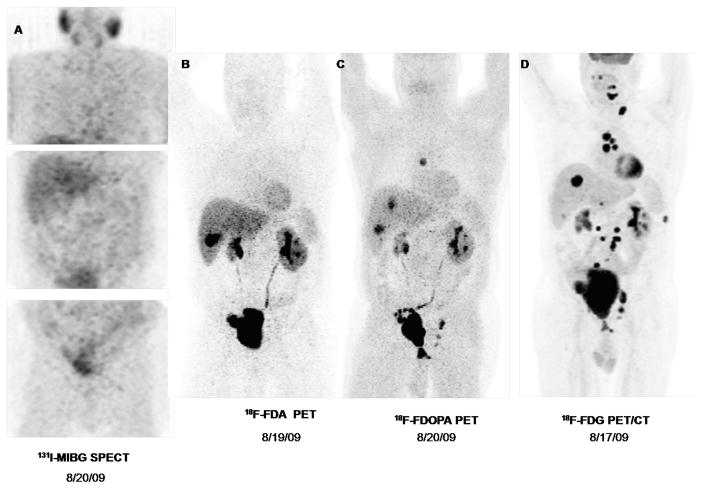
Among 13 patients with metastatic disease, metastases were noted in the lungs (n=8), multiple bony sites (n=9), lymph nodes (n=12), liver (n=5), and other soft tissues (n=3). Despite multiple tumor sites found per patient, none showed avid 123I-MIBG uptake. Patient no. 10 with metastatic disease was concomitantly found to have another primary tumor in the right adrenal gland while patient no. 9 developed another primary tumor in bilateral carotid bodies with metastatic disease to the liver, lungs, lymph nodes and bones as shown in Figure 1 after removal of a urinary bladder PGL. In these patients, both primary and metastatic tumors had no uptake of 123I-MIBG.
FIGURE 1.
Nuclear imaging studies of a 55-year old male with metastatic pheochromocytoma who tested negative for succinate dehydrogenase B, C, and D mutation. Primary tumor was found in the urinary bladder and was removed with en bloc cystectomy, prostatectomy, with lymphatic node dissection and creation of ileal neobladder in 2005. In 2009, he was diagnosed with metastatic disease with false-negative iodine-123 metaiodobenzylguanidine single photon emission computed tomography(123I-MIBG SPECT/CT). At NIH, 123I-MIBG SPECT/CT (A) was also negative but other nuclear imaging such as 18F-fluorodopamine (18F-FDA) (B), 18F-dihydroxyphenylalanine (18F-FDOPA) (C), and especially 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography (18F-FDG PET) (D) showed multiple metastatic foci in lymph nodes, lungs, and liver. He underwent radiofrequency ablation of liver lesions. A repeat 18F-FDG PET/CT after 3 months showed evidence of progression.
Of the 7 patients with adrenal PHEO (5 primary and non-metastatic; 1 recurrence; 1 new primary with metastases), the mean largest diameter of the tumor was 4.3 cm (range: 1.5 to 7 cm). In 1 patient with primary tumor on the right carotid body and in another patient with left paraaortic PGL, the tumor measured 0.8 cm and 1.5 cm, respectively.
Eleven (52%) patients were found to have SDHB mutation and 1 (4%) was found to have MEN 2 syndrome while the rest of the patients presented with apparently sporadic PHEO and/or PGL. Among SDHB-related tumors, 9 were associated with metastatic disease, 1 had multifocal tumor, and 1 had non-metastatic tumor.
Outcome Analysis
Patients with false-negative 123I-MIBG SPECT not later than 2008 were included for outcome analysis (n=19). Five patients were excluded due to lack of follow-up (n=4) and infection with human immunodeficiency virus (n=1). A total of 14 cases were analyzed with a mean follow-up period of 5 years (range: 2 – 7) after the first documented false-negative 123I-MIBG SPECT at NIH (Table 3). On initial presentation at NIH, 4 (29%) of these patients had no metastasis, while 10 (71%) had metastatic disease.
TABLE 3.
Outcome of Patients with False-Negative 123I-MIBG SPECT
| Living | Dead | |
|---|---|---|
|
| ||
| N | 9 | 5 |
|
| ||
| Tumor Type | ||
| Nonmetastatic | 4 | 0 |
| Metastatic | 5 | 5 |
|
| ||
| Primary Tumor Location | ||
| Adrenal | 2 | 2 |
| Extra-adrenal | 7 | 3 |
|
| ||
| Gene Mutation | ||
| SDHB | 5 | 4 |
| Sporadic | 4 | 1 |
Of the 4 patients with non-metastatic PHEO or PGL, none developed metastatic disease until March 2011 while 1 patient (patient no. 7) developed another primary tumor in the left carotid body and paraesophageal PGL after about 6 years following a false-negative 123I MIBG SPECT. Two of the non-metastatic cases were due to SDHB mutation while the other 2 were apparently sporadic. All patients are still alive.
Of the 10 patients with metastatic disease, 9 (90%) had SBHB mutation while the other 1 had apparently sporadic PHEO or PGL. Patient no.12 had multiple metastases to the lungs and left paraaortic lymph nodes which were excised. Patient no.15 had multiple metastases to the bones, liver and lymph nodes and was given chemotherapy with cyclophosphamide + vincristine + dacarbazine (CVD), sunitinib, bortezomib, 17-DMAG, and external beam radiotherapy. Patient no.16 who had multiple metastases to the bones, lungs, liver and lymph nodes received chemotherapy with CVD and underwent radiofrequency ablation of liver metastases. Patients no. 11 and 17 who had metastases to the bones, liver, lungs and lymph nodes were treated with CVD and 17-DMAG, respectively. Patient no. 18 had gamma knife radiotherapy of a carotid body tumor invading the skull which was later on resected. She also received CVD chemotherapy. Patient no. 19 eventually had metastatic lesions with avid uptake on subsequent 123I-MIBG SPECT and therefore underwent 131I-MIBG treatment but due to persistent disease, she also received temozolamide. Patient no. 21 had resection of the periaortic lymph node and hilar metastases and developed another primary tumor in the left adrenal gland 6 years after the initial diagnosis which was resected as well. Five (55%) (4 SDHB + 1 sporadic) died due to overwhelming metastases after a mean of 10.4 years (range: 3–17 years) and 5.2 years (range: 3–7 years) after the initial diagnosis of PHEO or PGL and development of metastatic disease, respectively. Sixty percent of deaths occurred within 5 years after the diagnosis of metastatic disease. Four other patients (2 SDHB + 2 sporadic) had progressive disease within a mean follow-up of 7.25 years (range: 6–9 years) while one other patient had stable metastatic disease.
DISCUSSION
The present study presents detailed characteristics and outcomes of patients with false-negative 123I-MIBG SPECT. Majority had primary tumors of extra-adrenal chromaffin tissues, noradrenergic biochemical phenotype, metastases, and over half had mutation in SDHB. The latter comprise 25% and 69% of patients with non-metastatic and metastatic PHEO or PGL, respectively. To our best knowledge, this is the first paper to show that a false-negative 123I-MIBG SPECT in patients with PHEO or PGL follow a more aggressive course and are frequently linked to the presence of SDHB mutation. In general, it is estimated that the rate of malignancy among patients with PHEO and PGL is about 10%–30% and that about 10% have metastatic disease upon initial presentation (Ilias et al. 2004, Goldstein et al. 1999). It is also estimated that about one third or more of PHEOs and PGLs have a defined genetic cause (Amar et al. 2005, Erlic et al. 2005, Mannelli et al. 2009). Nevertheless, in the present study, we have shown that among patients with false-negative 123I-MIBG SPECT, the rate of malignancy was higher at 62% (13 out of 21) and 24% (5 out of 21) had metastatic disease on initial presentation. Furthermore, 57% (12 out of 21) were due to a genetic mutation, with 92% (11 out of 12) of these cases being linked to SDHB mutation.
It is well established that PHEO or PGL due to SDHB mutation is associated with shorter survival (Amar et al. 2007) and higher incidence of malignancy (Timmers et al. 2009, Brouwers et al. 2006, Burnichon et al. 2009). It is estimated that the malignancy rate of SDHB-related PHEO or PGL is at least 30% and often much higher and the 5-yr probability of survival after the diagnosis of first metastasis among these patients drop to 36% in contrast to 67% among patients without that mutation (Amar et al. 2007). In our study, SDHB mutation accounted for 69% of metastatic disease and 80% of mortality.
In the present study, 44% (4 out of 9) of patients with apparently sporadic PHEO and PGL were found to have metastatic disease resulting in death in 1 patient within 6 years after the diagnosis of metastasis. In contrast to these data, it was previously shown that the rate of malignancy among apparently sporadic tumor is only 9% (Bravo and Tagle 2003). It should be further emphasized that of the 5 patients who had metastatic disease upon initial diagnosis, 3 had apparently sporadic tumor. These suggest that negative 123I-MIBG SPECT points toward unfavourable outcome which may be independent of SDHB mutation.
There are several factors that can cause a false-negative 123I-MIBG study. Medications such as labetalol, reserpine, calcium channel blockers, and antidepressants may interfere with MIBG uptake (Solanski et al. 1992; Havekes et al. 2008). 123/131I-MIBG scintigraphy has been shown to have lower sensitivity for small tumors (van der Harst et al. 2001, Bhatia et al. 2008, Wiseman et al. 2009). However, 2 of our patients had adrenal PHEOs measuring 7 cm with false-negative scan by 123I-MIBG SPECT. Although the sensitivity of 131/123I-MIBG scintigraphy is also generally suboptimal for extra-adrenal PGLs, we have also included in this series a patient with mediastinal PGL measuring 8 cm that had false-negative 123I-MIBG SPECT. The suboptimal sensitivity of 123/131I-MIBG for metastatic PHEO and PGL has also been shown in previous studies and is related to the dedifferentiation that results in loss of NETs among these tumors (Timmers et al. 2009, Ilias et al. 2003). The limited sensitivity of 123/131I-MIBG scintigraphy for familial cases of PHEO and PGL has also been documented (van der Harst et al. 2001, Bhatia et al. 2008 Taïeb et al. 2004, Ilias et al. 2003, Fottner et al. 2010, Timmers et al. 2009). Thus, 123I-MIBG scintigraphy is not recommended for these patients and negativity may reflect aggressive behaviour. Studies have also shown that a predominant NE and NMN secretion predicts negative123I-MIBG scintigraphy which was also observed in the current study (van der Harst et al. 2001, Fottner et al. 2010). However, one study has shown that the uptake of MIBG was not correlated with the plasma or urinary catecholamine levels but with the amount of neurosecretory granules within the tumor (Bomanji et al. 1987). Recently, it was shown that the lack of VMAT-1 expression explains false-negativity for MIBG scintigraphy (Fottner et al. 2010).
The present study has some limitations. Most patients that were referred to NIH due to metastatic disease did not have preoperative 123/131I-MIBG scintigraphy. Furthermore, the data may be affected by referral bias and follow-up period for some patients seen at NIH for their primary tumors was relatively short to certainly conclude a non-metastatic disease. It is also recommended that a head to head comparison be made as to the outcomes of SDHB-related tumors with avid and false-negative 123I-MIBG SPECT.
In conclusion, we have shown that a false-negative 123I-MIBG SPECT is frequently related to metastatic tumors, usually due to SDHB mutations with unfavourable prognosis. We, therefore, recommend that patients with false-negative 123I-MIBG SPECT be tested for SDHB mutations and should undergo more regular and close follow-up.
Acknowledgments
FUNDING
This work was supported by the Intramural Research Program of the National Institutes of Child Health and Human Development/National Institutes of Health.
Jay S. Fonte, M.D. and Jeremyjones F. Robles M.D. acknowledge the staff of the Section of Endocrinology and Metabolism, Department of Medicine, University of Santo Tomas Hospital, Manila, Philippines and the Thomasian Endocrine Progress Inc. for their invaluable support during their training at the National Institutes of Health.
Footnotes
DECLARATIONS OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
AUTHOR CONTRIBUTIONS
All authors were involved in conceptualizing this research paper and contributed in the writing and editing of the final manuscript.
Furthermore, Jay S. Fonte, M.D. and Jeremyjones F. Robles M.D. were involved in data gathering. Jay S. Fonte, M.D., Karel Pacak, M.D., Ph.D., D.Sc., Clara C. Chen, M.D., James Reynolds, M.D., and Millie Whatley reviewed the nuclear imaging studies of the patients. Jay S. Fonte M.D., Karel Pacak, M.D., Ph.D., D.Sc., and Alexander Ling, M.D. reviewed the CT scan and MRI of the patients. Karel Pacak, M.D., Ph.D., D.Sc., Tito Fojo, M.D., Karen Adams, Jay S. Fonte, M.D. and Jeremyjones F. Robles M.D were involved in the management of the patients included in the study. Jay S. Fonte, M.D. and Karel Pacak, M.D., Ph.D., D.Sc. wrote the initial manuscript.
References
- Amar L, Baudin E, Burnichon N, Peyrard S, Silvera S, Bertherat J, Bertagna X, Schlumberger M, Jeunemaitre X, Gimenez-Roqueplo AP, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. Journal of Clinical Endocrinology and Metabolism. 2007;92:3822–3828. doi: 10.1210/jc.2007-0709. [DOI] [PubMed] [Google Scholar]
- Amar L, Bertherat J, Baudin E, Ajzenberg C, Bressac-de Paillerets B, Chabre O, Chamontin B, Delemer B, Giraud S, Murat A, et al. Genetic testing in pheochromocytoma or functional paraganglioma. Journal of Clinical Oncology. 2005;23:8812–8818. doi: 10.1200/JCO.2005.03.1484. [DOI] [PubMed] [Google Scholar]
- Bhatia KS, Ismail MM, Sahdev A, Rockall AG, Hogarth K, Canizales A, Avril N, Monson JP, Grossman AB, Reznek RH. 123I-metaiodobenzylguanidine (MIBG) scintigraphy for the detection of adrenal and extra-adrenal phaeochromocytomas: CT and MRI correlation. Clinical Endocrinology (Oxf) 2008;69:181–188. doi: 10.1111/j.1365-2265.2008.03256.x. [DOI] [PubMed] [Google Scholar]
- Bomanji J, Levison DA, Flatman WD, Horne T, Bouloux PM, Ross G, Britton KE, Besser GM. Uptake of iodine-123 MIBG by pheochromocytomas, paragangliomas, and neuroblastomas: a histopathological comparison. Journal of Nuclear Medicine. 1987;28:973–978. [PubMed] [Google Scholar]
- Bravo EL, Tagle R. Pheochromocytoma: state-of-the-art and future prospective. Endocrine Reviews. 2003;24:539–553. doi: 10.1210/er.2002-0013. [DOI] [PubMed] [Google Scholar]
- Brouwers FM, Einsenhofer G, Tao JJ, Kant JA, Adams KT, Marston Linehan W, Pacak K. High frequency of SDHB germline mutations in patients with malignant catecholamine-producing paragangliomas: implications for genetic testing. Journal of Clinical Endocrinology and Metabolism. 2006;91:4505–4509. doi: 10.1210/jc.2006-0423. [DOI] [PubMed] [Google Scholar]
- Burnichon N, Rohmer V, Amar L, Herman P, Leboulleux S, Darrouzet V, Niccoli P, Gaillard D, Chabrier G, Chabolle F, et al. The succinate dehydrogenase genetic testing in a large prospective series of patients with paragangliomas. Journal of Clinical Endocrinology and Metabolism. 2009;94:2817–2827. doi: 10.1210/jc.2008-2504. [DOI] [PubMed] [Google Scholar]
- Castellani MR, Seghezzi S, Chiesa C, Aliberti GL, Maccauro M, Seregni E, Orunesu E, Luksch R, Bombardieri E. (131)I-MIBG treatment of pheochromocytoma: low versus intermediate activity regimens of therapy. Quarterly Journal of Nuclear Medicine and Molecular Imaging. 2010;54:100–113. [PubMed] [Google Scholar]
- Comino-Méndez I, Gracia-Aznárez FJ, Schiavi F, Landa I, Leandro-García LJ, Letón R, Honrado E, Ramos-Medina R, Caronia D, Pita G, et al. Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nature Genetics. 2011;43:663–667. doi: 10.1038/ng.861. [DOI] [PubMed] [Google Scholar]
- DeLellis RA, Lloyd RV, Heitz PU, Eng C. World Health Organization. WHO Classification of tumours, pathology and genetics of tumours of endocrine organs. Vol. 2004. Lyon: IARC Press; 2004. [Google Scholar]
- Eisenhofer G. The role of neuronal and extraneuronal plasma membrane transporters in the inactivation of peripheral catecholamines. Pharmacology and Therapeutics. 2001;91:35–62. doi: 10.1016/s0163-7258(01)00144-9. [DOI] [PubMed] [Google Scholar]
- Erlic Z, Rybicki L, Peczkowska M, Golcher H, Kann PH, Brauckhoff M, Müssig K, Muresan M, Schäffler A, Reisch N, et al. Clinical predictors and algorithm for the genetic diagnosis of pheochromocytoma patients. Clinical Cancer Research. 2009;15:6378–6385. doi: 10.1158/1078-0432.CCR-09-1237. [DOI] [PubMed] [Google Scholar]
- Fiebrich HB, Brouwers AH, Kerstens MN, Pijl ME, Kema IP, de Jong JR, Jager PL, Elsinga PH, Dierckx RA, van der Wal JE, et al. 6-[F-18]Fluoro-L-dihydroxyphenylalanine positron emission tomography is superior to conventional imaging with (123)I-metaiodobenzylguanidine scintigraphy, computer tomography, and magnetic resonance imaging in localizing tumors causing catecholamine excess. Journal of Clinical Endocrinology and Metabolism. 2009;94:3922–3930. doi: 10.1210/jc.2009-1054. [DOI] [PubMed] [Google Scholar]
- Fottner C, Helisch A, Anlauf M, Rossmann H, Musholt TJ, Kreft A, Schadmand-Fischer S, Bartenstein P, Lackner KJ, Klöppel G, et al. 6-18F-fluoro-L-dihydroxyphenylalanine positron emission tomography is superior to 123I-metaiodobenzyl-guanidine scintigraphy in the detection of extraadrenal and hereditary pheochromocytomas and paragangliomas: correlation with vesicular monoamine transporter expression. Journal of Clinical Endocrinology and Metabolism. 2010;95:2800–2810. doi: 10.1210/jc.2009-2352. [DOI] [PubMed] [Google Scholar]
- Goldsmith SJ. Update on nuclear medicine imaging of neuroendocrine tumors. Future Oncology. 2009;5:75–84. doi: 10.2217/14796694.5.1.75. [DOI] [PubMed] [Google Scholar]
- Goldstein RE, O'Neill JA, Jr, Holcomb GW, Morgan WM, Neblett WW, Oates JA, Brown N, Nadeau J, Smith B, Page DL, et al. Clinical experience over 48 years with pheochromocytoma. Annals of Surgery. 1999;229:755–766. doi: 10.1097/00000658-199906000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonias S, Goldsby R, Matthay KK, Hawkins R, Price D, Huberty J, Damon L, Linker C, Sznewajs A, Shiboski S, et al. Phase II study of high-dose [131I]metaiodobenzylguanidine therapy for patients with metastatic pheochromocytoma and paraganglioma. Journal of Clinical Oncology. 2009;27:4162–4168. doi: 10.1200/JCO.2008.21.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havekes B, Lai EW, Corssmit EPM, Romijn JA, Timmers HJLM, Pacak K. Detection and treatment of pheochromocytomas and paragangliomas: current standing of MIBG scintigraphy and future role of PET imaging. Quarterly Journal of Nuclear Medicine and Molecular Imaging. 2008;52:419–429. [PubMed] [Google Scholar]
- Hensen EF, Bayley JP. Recent advances in the genetics of SDH-related paraganglioma and pheochromocytoma. Familial Cancer. 2011;10:355–363. doi: 10.1007/s10689-010-9402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilias I, Pacak K. Current approaches and recommended algorithm for the diagnostic localization of pheochromocytoma. Journal of Clinical Endocrinology and Metabolism. 2004;89:479–491. doi: 10.1210/jc.2003-031091. [DOI] [PubMed] [Google Scholar]
- Ilias I, Yu J, Carrasquillo JA, Chen CC, Eisenhofer G, Whatley M, McElroy B, Pacak K. Superiority of 6-[18F]-fluorodopamine positron emission tomography versus [131I]-metaiodobenzylguanidine scintigraphy in the localization of metastatic pheochromocytoma. Journal of Clinical Endocrinology and Metabolism. 2003;88:4083–4087. doi: 10.1210/jc.2003-030235. [DOI] [PubMed] [Google Scholar]
- Jacobson AF, Deng H, Lombard J, Lessig HJ, Black RR. 123I-meta-iodobenzylguanidine scintigraphy for the detection of neuroblastoma and pheochromocytoma: results of a meta-analysis. Journal of Clinical Endocrinology and Metabolism. 2010;95:2596–2606. doi: 10.1210/jc.2009-2604. [DOI] [PubMed] [Google Scholar]
- Kaji P, Carrasquillo JA, Linehan WM, Chen CC, Eisenhofer G, Pinto PA, Lai EW, Pacak K. The role of 6-[18F]fluorodopaminepositron emission tomography in the localization of adrenal pheochromocytoma associated with von Hippel-Lindau syndrome. European Journal of Endocrinology. 2007;156:483–487. doi: 10.1530/EJE-06-0712. [DOI] [PubMed] [Google Scholar]
- Kauhanen S, Seppänen M, Ovaska J. The clinical value of [18F]fluorodihydroxyphenylalanine positron emission tomography in primary diagnosis, staging, and restaging of neuroendocrine tumors. Endocrine-Related Cancer. 2009;16:255–265. doi: 10.1677/ERC-08-0229. [DOI] [PubMed] [Google Scholar]
- Loh KC, Fitzgerald PA, Matthay KK, Yeo PP, Price DC. The treatment of malignant pheochromocytoma with iodine-131 metaiodobenzylguanidine (131I-MIBG): a comprehensive review of 116 reported patients. Journal of Endocrinological Investigation. 1997;20:648–658. doi: 10.1007/BF03348026. [DOI] [PubMed] [Google Scholar]
- Maher ER, Eng C. The pressure rises: update on the genetics of phaeochromocytoma. Human Molecular Genetics. 2002;11:2347–2354. doi: 10.1093/hmg/11.20.2347. [DOI] [PubMed] [Google Scholar]
- Mannelli M, Castellano M, Schiavi F, Filetti S, Giacchè M, Mori L, Pignataro V, Bernini G, Giachè V, Bacca A, et al. Clinically guided genetic screening in a large cohort of Italian patients with pheochromocytomas and/or functional or nonfunctional paragangliomas. Journal of Clinical Endocrinology and Metabolism. 2009;94:1541–1547. doi: 10.1210/jc.2008-2419. [DOI] [PubMed] [Google Scholar]
- Meyer-Rochow GY, Schembri GP, Benn DE. The utility of metaiodobenzylguanidine single photon emission computed tomography/computed tomography (MIBG SPECT/CT) for the diagnosis of pheochromocytoma. Annals of Surgical Oncology. 2010;17:392–400. doi: 10.1245/s10434-009-0850-5. [DOI] [PubMed] [Google Scholar]
- Rose B, Matthay KK, Price D, Huberty J, Klencke B, Norton JA, Fitzgerald PA. High-dose 131I-metaiodobenzylguanidine therapy for 12 patients with malignant pheochromocytoma. Cancer. 2003;98:239–248. doi: 10.1002/cncr.11518. [DOI] [PubMed] [Google Scholar]
- Shulkin BL, Thompson NW, Shapiro B, Francis IR, Sisson JC. Pheochromocytomas: imaging with 2-[fluorine-18]fluoro-2-deoxy-D-glucose PET. Radiology. 1999;212:35–41. doi: 10.1148/radiology.212.1.r99jl3035. [DOI] [PubMed] [Google Scholar]
- Sisson JC, Frager MS, Valk TW, Gross MD, Swanson DP, Wieland DM, Tobes MC, Beierwaltes WH, Thompson NW. Scintigraphic localization of pheochromocytoma. New England Journal of Medicine. 1981;305:12–17. doi: 10.1056/NEJM198107023050103. [DOI] [PubMed] [Google Scholar]
- Solanki KK, Bomanji J, Moyes J, Mather SJ, Trainer PJ, Britton KE. A pharmacological guide to medicines which interfere with the biodistribution of radiolabelled metaiodobenzylguanidine (MIBG) Nuclear Medicine Communications. 1992;13:513–521. doi: 10.1097/00006231-199207000-00006. [DOI] [PubMed] [Google Scholar]
- Taïeb D, Sebag F, Hubbard JG, Mundler O, Henry JF, Conte-Devolx B. Does iodine-131 meta-iodobenzylguanidine (MIBG) scintigraphy have an impact on the management of sporadic and familial phaeochromocytoma? Clinical Endocrinology (Oxf) 2004;61:102–108. doi: 10.1111/j.1365-2265.2004.02077.x. [DOI] [PubMed] [Google Scholar]
- Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, Eisenhofer G, Martiniova L, Adams KT, Pacak K. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. Journal of Clinical Endocrinology and Metabolism. 2009;94:4757–4767. doi: 10.1210/jc.2009-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers HJ, Eisenhofer G, Carrasquillo JA, Chen CC, Whatley M, Ling A, Adams KT, Pacak K. Use of 6-[18F]-fluorodopamine positron emission tomography(PET) as first-line investigation for the diagnosis and localization of non-metastatic and metastatic phaeochromocytoma (PHEO) Clinical Endocrinology (Oxf) 2009;71:11–17. doi: 10.1111/j.1365-2265.2008.03496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers HJ, Kozupa A, Chen CC, Carrasquillo JA, Ling A, Eisenhofer G, Adams KT, Solis D, Lenders JW, Pacak K. Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. Journal of Clinical Oncology. 2007;25:2262–2269. doi: 10.1200/JCO.2006.09.6297. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan G. Meta-iodobenzylguanidine and analogues: chemistry and biology. Quarterly Journal of Nuclear Medicine and Molecular Imaging. 2008;52:351–368. [PubMed] [Google Scholar]
- van der Harst E, de Herder WW, Bruining HA, Bonjer HJ, de Krijger RR, Lamberts SW, van de Meiracker AH, Boomsma F, Stijnen T, Krenning EP, et al. [(123)I]Metaiodobenzylguanidine and [(111)In]octreotide uptake in benign and malignant pheochromocytomas. Journal of Clinical Endocrinology and Metabolism. 2001;86:685–693. doi: 10.1210/jcem.86.2.7238. [DOI] [PubMed] [Google Scholar]
- Wiseman GA, Pacak K, O'Dorisio MS, Neumann DR, Waxman AD, Mankoff DA, Heiba SI, Serafini AN, Tumeh SS, Khutoryansky N, et al. Usefulness of 123I-MIBG scintigraphy in the evaluation of patients with known or suspected primary or metastatic pheochromocytoma or paraganglioma: results from a prospective multicenter trial. Journal of Nuclear Medicine. 2009;50:1448–1454. doi: 10.2967/jnumed.108.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]