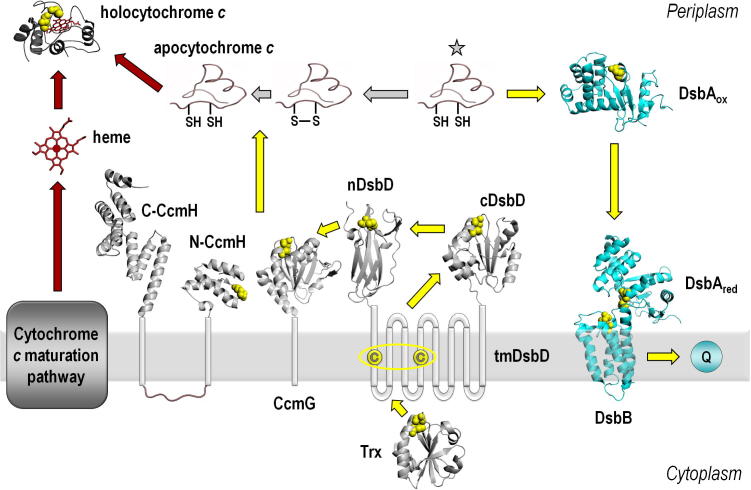
Fig. 1.
Scheme illustrating the proteins affecting the oxidation state of the CXXCH motif of the apocytochrome before covalent heme attachment in the bacterial periplasm. Apocytochrome enters the periplasm in a reduced state (both cysteines in a thiol form, indicated with a star) and can be oxidized by the protein DsbA which then transfers the acquired electrons to the membrane protein DsbB and itself is restored to its active oxidized state. DsbB passes the electrons to the respiratory chain through quinone (Q). The oxidized apocytochrome (disulfide bond between the cysteines of the CXXCH motif) is reduced by proteins CcmG and/or CcmH of the Ccm system. The necessary reducing power originates from cytoplasmic thioredoxin (Trx). Reductant is transferred sequentially to tmDsbD, cDsbD and nDsbD; the latter is the reductant provider for several periplasmic pathways. The reduced apocytochrome can then have heme attached by the heme-handling proteins CcmA–F. Yellow arrows indicate electron flow and red arrows indicate steps involving heme handling. Cysteines are depicted as yellow spheres. The PDB entries used are 2TRX, 1FVK, 2HI7, 2FWH, 1JPE, 2BLK, 2E2E, and 155C. Structures were rendered in Pymol (DeLano, W.L. The Pymol Molecular Graphics System (2002) http://www.pymol.org).