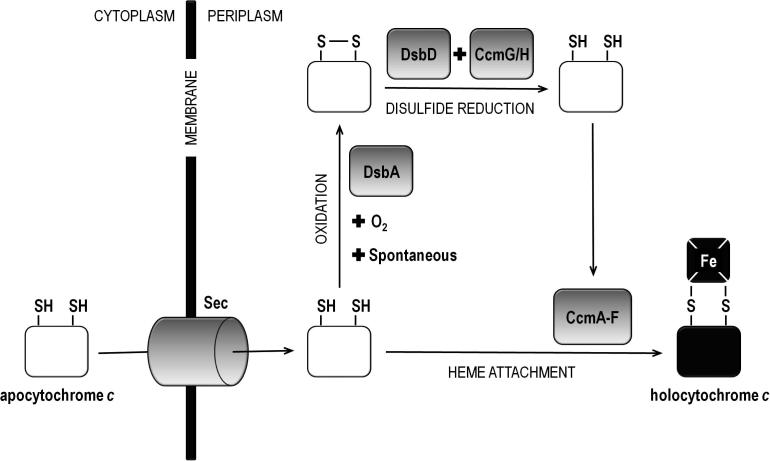
Fig. 2.
Model illustrating the involvement of DsbA, DsbD and the Ccm system in the formation of holocytochrome c in E. coli. Upon entering into the periplasm, through the Sec system, the reduced and unfolded apocytochrome can undergo the competing processes of either heme attachment to, or oxidation of, its cysteine thiols. A fraction of the apoprotein interacts with the Ccm heme attachment proteins (CcmA–F) directly. Another fraction instead is oxidized by with DsbA (or oxygen when present). Then it is dependent on the presence of the reducing proteins DsbD and CcmG/H, which eventually deliver it in the reduced state to CcmA–F, for heme attachment. The final product, holocytochrome c, can be produced via both pathways.