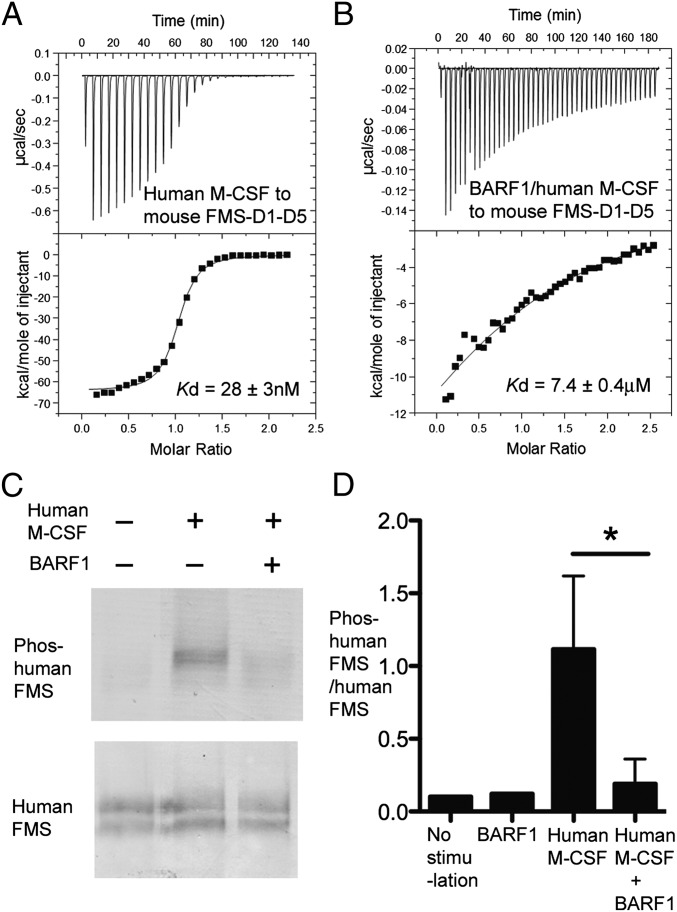
Fig. 1.
BARF1 reduces M-CSF:receptor binding and M-CSF-induced receptor phosphorylation. (A and B) Calorimetric measurements of the binding between human M-CSF (A) and mouse FMS (B) ECD in the absence vs. presence of BARF1. The affinities are shown by the fitted curves. (C) Representative blot of cell-based phosphorylation assays showing that human M-CSF induces the phosphorylation of human FMS Tyr723 much more efficiently in the absence than in the presence of BARF1. COS7 cells expressing human FMS were stimulated with 75 nM of M-CSF or BARF1:M-CSF for 2 min. Cell lysates were analyzed with antibodies against M-CSF receptor and phospho-M-CSF receptor (Tyr723) (D) Quantification of the cell-based phosphorylation assay represented in C. Blots were scanned, and band intensities were quantified using ImageJ 1.45p software. Data are represented as ratios of Tyr723-phosphorylated human FMS to total human FMS levels as challenged by different stimuli, showing that BARF1 efficiently reduces M-CSF signaling but does not reduce signaling to the basal level when no ligand is present. Data for “No stimulation” and “BARF1” represent one experiment; the remaining data represent the mean and SD of three independent experiments. *P < 0.05 using Student’s t test.