Abstract
Coronary artery fistulas are rare anomalies of the coronary arteries that may sometimes cause symptoms by shunting blood flow away from the myocardial capillary network. We report the case of a 46-year old lady which shows the right coronary cusp giving rise to left main coronary artery called anomalous origin of a coronary artery (AOCA), and also a fistula between the left coronary artery and pulmonary artery. We describe our diagnostic approach and review the literature on the epidemiology, pathophysiology, the diagnostic modalities, and treatment options.
1. Introduction
Coronary artery fistula (CAF) is defined as an anomalous connection between a coronary artery and a major vessel or a cardiac chamber. It is an uncommon form of congenital heart disease. Most of the coronary anomalies are incidental findings during angiographic evaluation for coronary vascular disorders. Majority of these fistulas arise from the left anterior descending artery or from the right coronary artery [1]. Most of these patients are asymptomatic, but heart failure, angina, myocardial infarction, coronary steal, endocarditis, and dyspnea have been reported in some cases [2]. The management is complicated, and recommendations are based on anecdotal cases of very small retrospective series. We present a case of CAF with coronary anomaly.
2. Epidemiology
The incidence of coronary anomalies varies between 0.6% and 1.5% of patients undergoing invasive cardiovascular imaging [3, 4]. CAF was first described in 1841, and it is defined as an anomalous connection between a coronary artery and a major vessel or cardiac chamber [5]. Its incidence in the general population is about 0.002%. The incidence of coronary pulmonary fistulas was similarly reported only to be 0.1% in a study of 11, 000 patients undergoing cardiac catheterization [6].
3. Anatomy
AOCA arising from the opposite sinus of Valsalva (either the right coronary artery arising from the left sinus or the left coronary artery arising from the right sinus of Valsalva) especially an anomalous coronary artery coursing between the great arteries has been associated with myocardial ischemia, ventricular arrhythmias, and sudden death, (Figure 1) [7–26]. Two forms of AOCA not associated with myocardial ischemia or sudden death are AOCA from the noncoronary or posterior sinus of Valsalva [9, 10, 26]. The interarterial course of anomalous coronary artery (ACA) can be between the myocardial sulcus and the great arteries (intramyocardial) [15, 26] or within the anterior wall of the aorta between the great arteries (intramural) [7, 26].
Figure 1.
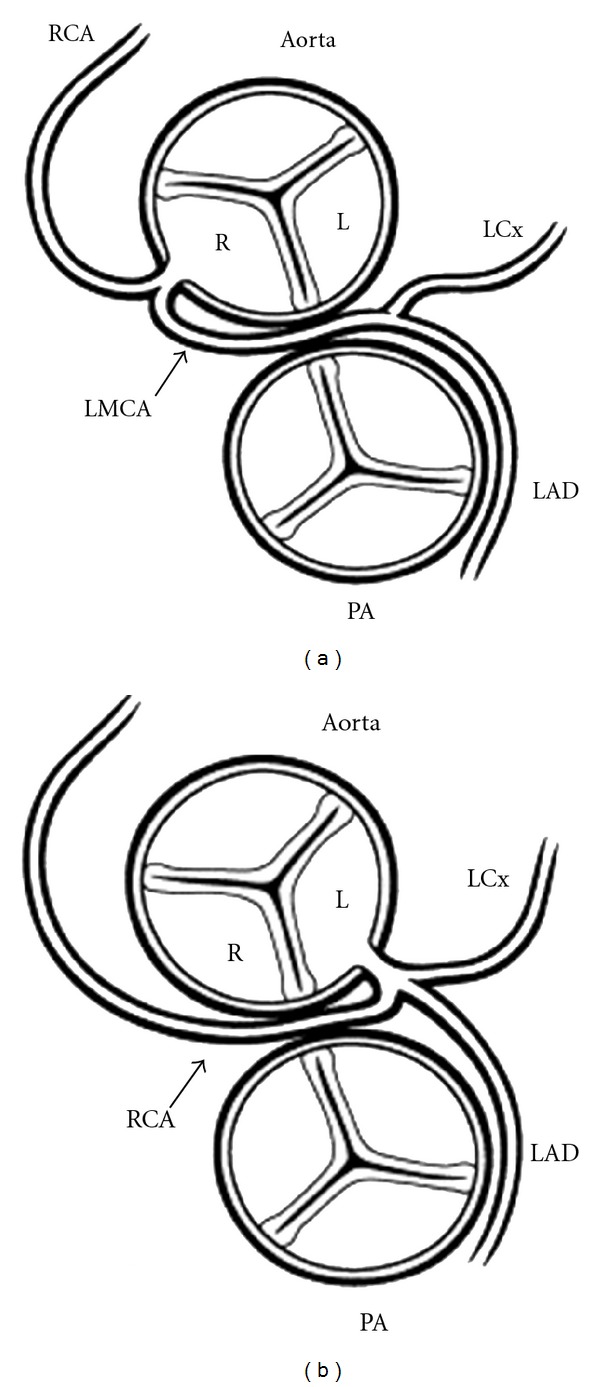
Schematic diagram of the two forms of anomalous origin of a coronary from the wrong sinus. Those are associated with myocardial ischemia. (a) Anomalous origin of the left coronary artery (LCA) from the right sinus of Valsalva. With anomalous origin of the left coronary, the left main coronary artery (LMCA) arises from the right aortic sinus (R) and passes between the great arteries before dividing into its two usual branches, the left anterior descending (LAD) and left circumflex (LCx) coronary arteries. (b) Anomalous origin of the right coronary artery (RCA) from the left sinus of Valsalva. With anomalous origin of the right coronary, the right coronary artery (RCA) arises from the left aortic sinus (L) and passes between the great arteries before coursing in its usual distribution. [26]. In each case, the anomalous coronary can be seen coursing between the aorta and pulmonary artery (PA).
The exact mechanisms that lead to myocardial ischemia in the patients with AOCA from the opposite sinus that course between the pulmonary and aortic roots are unclear, but several theories have been proposed. The anomalous coronary artery usually arises at an acute angle from the aorta, rather than perpendicularly; this may alter flow patterns into that coronary artery bed [15, 26]. Another proposed mechanism is compromised flow reserve secondary to slit like ostium associated with ACA [9, 26]. Finally, it was hypothesized that the interarterial course places the anomalous coronary at risk of compression between the great arteries. The ischemia could be from deformation of the anomalous coronary within the aortic wall during periods of systemic hypertension, particularly in patients who have an intramural course [26]. Because wall tension is determined by the radius of a vessel, the aorta will have greater wall tension than the intramural coronary within the aortic wall which results in deformation of the coronary and diminished cross-sectional area. During exercise, aortic wall tension increases, and with increasing aortic pressure the anomalous coronary becomes flattened and coronary reserve is reduced to a point where myocardial oxygen requirements are not met.
4. Pathophysiology
When the fistula drains into the right side of the heart, the volume load is increased to the right heart as well as to the pulmonary vascular bed, the left atrium, and left ventricle. When the fistula drains into the left atrium or the left ventricle, there is volume overloading of these chambers but no increase in the pulmonary blood flow. This results in varied appearance of dilation of different cardiac chambers due to the shunts on echocardiography. The size of the shunt is determined by the size of the fistula and the pressure difference between the coronary artery and the chamber into which the fistula drains. Occasionally, congestive cardiac failure occurs, and vey rarely, in adults, myocardial ischemia may occur [27, 28].
5. Case Report
A 46-year old, lady with past medical history significant for dyslipidemia and gastroesophageal reflux presents to her primary care physician with complaint of chest pain. Pain is described as 8/10, retrosternal, compressive pain, radiating to her neck bilaterally, intermittent in nature, and lasting for nine months. Patient mainly complained of pain during the day, while working, and had occasional relief when she was resting. Pain was associated with palpitations and shortness of breath and had an average of 2-3 episodes per week lasting 10–15 minutes each. No other symptoms were reported at that time. Patient denied having any family history of coronary artery disease or history of alcohol, tobacco, and illicit drug usage. Patient was proceeded to get an EKG and stress test. EKG did not show any acute abnormalities and Thallium stress test showed a mild degree of myocardial ischemia involving basal region of anterior wall, which was reversible. A cardiac catheterization was done, and it revealed left main coronary artery arising from the right cusp (Figure 1(a)) and a fistula between the left coronary and pulmonary artery (Figure 1(b)). Cardiothoracic surgery was consulted, and a ligation of the coronary artery fistula was done. On reevaluation of the patient 3 months after procedure, patient remains chest-pain-free and continues followup at the cardiology clinic.
6. Coronary Artery Fistulas
Congenital coronary artery fistula (CAF) is a rare, isolated anomaly of the coronary artery system that is defined as a direct communication between a coronary artery and another vascular structure. The involved coronary usually arises normally from the aorta and connects to one of the intracardiac chambers, systemic veins, or pulmonary artery by way of a tortuous anomalous branch. Fistulas more frequently involve the right coronary artery and usually drain into one of the right heart chambers [29]. Symptoms and signs are dependent on the size of the fistulous connection; rarely, large fistulas can have a significant left-to-right shunt with resultant congestive heart failure and cardiomegaly in infancy [30]. Most patients who have CAF are asymptomatic in childhood and present because of a continuous murmur that is appreciated along the precordium. This murmur can sound similar to a patent ductus arteriosus, although its parasternal location frequently suggests a different etiology. Late complications have been described in older adults and include bacterial endocarditis, congestive heart failure, and angina [29, 31, 32]. Two-dimensional and color Doppler echocardiography usually are diagnostic and can identify the involved coronary and its site of drainage [33]. Surgical closure is safe and effective [34, 35]. Recent advances in interventional techniques now allow closure of CAF in the cardiac catheterization laboratory with the use of detachable coils and balloons; this has become the initial treatment of choice in children and adults [36–38]. Closure of the fistula is indicated when symptoms are present; the need for prophylactic closure in asymptomatic children to prevent late complications remains controversial.
7. Diagnostic Evaluation
Coronary angiography remains the gold standard for diagnosis. Angiography helps define the artery of origin, recipient vessel, or chamber and the site of communication. Noninvasive techniques such as transthoracic echocardiography, transesophageal echocardiography, and magnetic resonance imaging are becoming increasingly popular for diagnosis and followup of CAFs. Combined two-dimensional and pulsed Doppler echocardiography demonstrates a dilated coronary artery, turbulent flow in the fistula and the recipient chamber [27, 28]. Transthoracic color Doppler echocardiography with a high-frequency transducer and a low Nyquist limit allows multiple coronary arteries—left ventricular microfistulae to be visualized. Transesophageal echocardiography is used intraoperatively to identify the precise location of the site of drainage of the fistula, which could not be accurately revealed with preoperative coronary arteriography [39]. Magnetic resonance imaging and multidetector computed tomography have also become the alternative methods to evaluate the anatomy, flow, and function of CAF [27, 28, 40, 41].
8. Management
The natural history of coronary artery fistulas is variable. Spontaneous closure secondary to spontaneous thrombosis, although uncommon, has been reported [42, 43]. The management is controversial, and recommendations are based on anecdotal cases or small retrospective series [44]. Antiplatelet therapy is recommended, especially in patients with distal coronary artery fistulas and abnormally dilated coronary arteries [44]. Prophylactic precautions against subacute bacterial endocarditis are recommended, as bacterial endocarditis is a known complication.
The main indications for closure are clinical symptoms especially of heart failure and myocardial ischemia and in asymptomatic patients with high-flow shunting, to prevent occurrence of symptoms or complications, especially in pediatric population [45]. Surgery and direct epicardial or endocardial ligations were traditionally viewed as the main therapeutic method for the closure of coronary artery fistulas [45]. Surgical correction is safe and effective, with good results [34, 45, 46]. Treatment of adult asymptomatic patients with nonsignificant shunting is still a matter of debate.
Catheter-based closure of the fistulous connection is the nonsurgical treatment option for closure of coronary fistulas, with good reported success [47–50]. Since its introduction in the early 1980s, transcatheter closure of coronary artery fistulas is now widely available and for nearly a decade is being recognized as an effective and safe treatment option for coronary artery fistulas [50]. Catheter closure can be performed with a variety of techniques, including detachable balloons, stainless steel coils, controlled-release coils, controlled-release patent ductus arteriosus coils, patent ductus arteriosus plug, regular and covered stents, and various chemicals [46–52]. The vast majority of the fistulas treated with catheter intervention were occluded with coils. Controlled-release coils have made an important contribution to the technique of catheter closure of coronary artery fistulas [34]. The treated vessel after occlusion becomes thrombosed to the level of first branch and the reduction in left to right shunt causes myocardial perfusion return to normal [47]. Results from the transcatheter and surgical literature show that both approaches have similar early effectiveness, morbidity, and mortality [50]. The safe and effective results of both approaches support the option for elective closure of clinically significant coronary artery fistulas in childhood [50]. Occasionally, transcatheter-based stent implantation in a restrictive coronary-pulmonary fistula is needed, to augment pulmonary flow as a palliative management option in the adult patient with complex congenital heart disease, such as pulmonary atresia, ventricular septal defect with exercise intolerance and severe cyanosis [53].
9. Summary
Congenital coronary artery abnormalities are rare, isolated anomalies that are important to recognize in childhood. Usually, isolated coronary anomalies are asymptomatic; however, certain forms are associated with myocardial ischemia, congestive heart failure, and sudden cardiac death in infants and children. Recognition of signs and symptoms that may indicate a congenital coronary artery anomaly should lead to additional testing, especially thorough evaluation of coronary artery anatomy using two-dimensional and color Doppler echocardiography.
Disclosure
All of the authors have read and approved the final manuscript prior to submission. Further, the authors have no commercial or proprietary interest in any drug, device, or equipment mentioned in the submitted paper that may interfere with the process of submitting the paper to journal.
References
- 1.Raju MG, Goyal SK, Punnam SR, Shah DO, Smith GF, Abela GS. Coronary artery fistula: a case series with review of the literature. Journal of Cardiology. 2009;53(3):467–472. doi: 10.1016/j.jjcc.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Vijayvergiya R, Bhadauria PS, Jeevan H, Mittal BR, Grover A. Myocardial ischemia secondary to dual coronary artery fistulas draining into main pulmonary artery. International Journal of Cardiology. 2010;140(2):e30–e33. doi: 10.1016/j.ijcard.2008.11.074. [DOI] [PubMed] [Google Scholar]
- 3.Kardos A, Babai L, Rudas L, et al. Epidemiology of congenital coronary artery anomalies: a coronary arteriography study on a Central European population. Catheterization and Cardiovascular Diagnosis. 1997;42(3):270–275. doi: 10.1002/(sici)1097-0304(199711)42:3<270::aid-ccd8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Malouf JF, Edwards WD, Tajik AJ, Seward JB. Functional anatomy of the heart. In: Fuster VAR, editor. Hurst's the Heart. 11th edition. New York, NY, USA: McGraw-Hill; 2004. [Google Scholar]
- 5.Fernandes ED, Kadivar H, Hallman GL, Reul GJ, Ott DA, Cooley DA. Congenital malformations of the coronary arteries: the Texas Heart Institute experience. Annals of Thoracic Surgery. 1992;54(4):732–740. doi: 10.1016/0003-4975(92)91019-6. [DOI] [PubMed] [Google Scholar]
- 6.Said SA, Landman GH. Coronary-pulmonary fistula: long-term follow-up in operated and non-operated patients. International Journal of Cardiology. 1990;27(2):203–210. doi: 10.1016/0167-5273(90)90161-w. [DOI] [PubMed] [Google Scholar]
- 7.Frommelt PC, Berger S, Pelech AN, Bergstrom S, Williamson JG. Prospective identification of anomalous origin of left coronary artery from the right sinus of Valsalva using transthoracic echocardiography: importance of color Doppler flow mapping. Pediatric Cardiology. 2001;22(4):327–332. doi: 10.1007/s002460010239. [DOI] [PubMed] [Google Scholar]
- 8.Liberthson RR, Dinsmore RE, Fallon JT. Aberrant coronary artery origin fron the aorta. Report of 18 patients, review of literature and delineation of natural history and management. Circulation. 1979;59(4):748–754. doi: 10.1161/01.cir.59.4.748. [DOI] [PubMed] [Google Scholar]
- 9.Roberts WC. Major anomalies of coronary arterial origin seen in adulthood. American Heart Journal. 1986;111(5):941–963. doi: 10.1016/0002-8703(86)90646-0. [DOI] [PubMed] [Google Scholar]
- 10.Frescura C, Basso C, Thiene G, et al. Anomalous origin of coronary arteries and risk of sudden death: a study based on an autopsy population of congenital heart disease. Human Pathology. 1998;29(7):689–695. doi: 10.1016/s0046-8177(98)90277-5. [DOI] [PubMed] [Google Scholar]
- 11.Taylor AJ, Rogan KM, Virmani R. Sudden cardiac death associated with isolated congenital coronary artery anomalies. Journal of the American College of Cardiology. 1992;20(3):640–647. doi: 10.1016/0735-1097(92)90019-j. [DOI] [PubMed] [Google Scholar]
- 12.Lipsett J, Byard RW, Carpenter BF, Jimenez CL, Bourne AJ. Anomalous coronary arteries arising from the aorta associated with sudden death in infancy and early childhood. Archives of Pathology and Laboratory Medicine. 1991;115(8):770–773. [PubMed] [Google Scholar]
- 13.Kragel AH, Roberts WC. Anomalous origin of either the right or left main coronary artery from the aorta with subsequent coursing between aorta and pulmonary trunk: analysis of 32 necropsy cases. American Journal of Cardiology. 1988;62(10):771–777. doi: 10.1016/0002-9149(88)91220-9. [DOI] [PubMed] [Google Scholar]
- 14.Herrmann MA, Dousa MK, Edwards WD. Sudden infant death with anomalous origin of the left coronary artery. American Journal of Forensic Medicine and Pathology. 1992;13(3):191–195. doi: 10.1097/00000433-199209000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Roberts WC, Shirani J. The four subtypes of anomalous origin of the left main coronary artery from the right aortic sinus (or from the right coronary artery) American Journal of Cardiology. 1992;70(1):119–121. doi: 10.1016/0002-9149(92)91406-t. [DOI] [PubMed] [Google Scholar]
- 16.Barth CW, Roberts WC. Left main coronary artery originating from the right sinus of Valsalva and coursing between the aorta and pulmonary trunk. Journal of the American College of Cardiology. 1986;7(2):366–373. doi: 10.1016/s0735-1097(86)80507-1. [DOI] [PubMed] [Google Scholar]
- 17.Aoyagi S, Suzuki S, Kosuga K, Ohishi K. Anomalous origin of the right coronary artery associated with congenital bicuspid aortic valve. Kurume Medical Journal. 1991;38(3):199–202. doi: 10.2739/kurumemedj.38.199. [DOI] [PubMed] [Google Scholar]
- 18.Roberts WC, Siegel RJ, Zipes DP. Origin of the right coronary artery from the left sinus of Valsalva and its functional consequences: analysis of 10 necropsy patients. The American Journal of Cardiology. 1982;49(4):863–868. doi: 10.1016/0002-9149(82)91970-1. [DOI] [PubMed] [Google Scholar]
- 19.Isner JM, Shen EM, Martin ET, Fortin RV. Sudden unexpected death as a result of anomalous origin of the right coronary artery from the left sinus of Valsalva. American Journal of Medicine. 1984;76(1):155–158. doi: 10.1016/0002-9343(84)90765-4. [DOI] [PubMed] [Google Scholar]
- 20.Berdoff R, Haimowitz A, Kupersmith J. Anomalous origin of the right coronary artery from the left sinus of valsalva. American Journal of Cardiology. 1986;58(7):656–657. doi: 10.1016/0002-9149(86)90298-5. [DOI] [PubMed] [Google Scholar]
- 21.McManus BM, Gries LA, Ness MJ, Galup LN. Anomalous origin of the right coronary artery from the left sinus of valsalva. Pediatric Pathology. 1990;10(6):987–991. doi: 10.3109/15513819009064732. [DOI] [PubMed] [Google Scholar]
- 22.Barth CW, Bray M, Roberts WC. Sudden death in infancy associated with origin of both left main and right coronary arteries from a common ostium above the left sinus of Valsalva. American Journal of Cardiology. 1986;57(4):365–366. doi: 10.1016/0002-9149(86)90931-8. [DOI] [PubMed] [Google Scholar]
- 23.Liberthson RR, Gang DL, Custer J. Sudden death in an infant with aberrant origin of the right coronary artery from the left sinus of valsalva of the aorta: case report and review of the literature. Pediatric Cardiology. 1983;4(1):45–48. doi: 10.1007/BF02281006. [DOI] [PubMed] [Google Scholar]
- 24.Mantovani E, Carraro R, Thiene G. Once again on juvenile sudden death due to anomalous origin of the right coronary artery from the left anterior sinus of Valsalva. A case report. Italian Journal of Cardiology. 1987;17(II):791–794. [PubMed] [Google Scholar]
- 25.Hanzlick R, Stivers RR. Sudden death in a marathon runner with origin of the right coronary artery from the left sinus of Valsalva. The American Journal of Cardiology. 1983;51(8):p. 1467. doi: 10.1016/0002-9149(83)90337-5. [DOI] [PubMed] [Google Scholar]
- 26.Frommelt PC, Frommelt MA. Congenital coronary artery anomalies. Pediatric Clinics of North America. 2004;51(5):1273–1288. doi: 10.1016/j.pcl.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Qureshi SA. Coronary arterial fistulas. Orphanet Journal of Rare Diseases. 2006;1:p. 51. doi: 10.1186/1750-1172-1-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong GR, Choi SH, Kang SM, et al. Multiple coronary artery-left ventricular microfistulae in a patient with apical hypertrophic cardiomyopathy: a demonstration by transthoracic color Doppler echocardiography. Yonsei Medical Journal. 2003;44(4):710–714. doi: 10.3349/ymj.2003.44.4.710. [DOI] [PubMed] [Google Scholar]
- 29.Lowe JE, Oldham HN, Sabiston DC. Surgical management of congenital coronary artery fistulas. Annals of Surgery. 1981;194(4):373–379. doi: 10.1097/00000658-198110000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsieh KS, Huang TC, Lee CL. Coronary artery fistulas in neonates, infants, and children: clinical findings and outcome. Pediatric Cardiology. 2002;23(4):415–419. doi: 10.1007/s00246-002-1334-6. [DOI] [PubMed] [Google Scholar]
- 31.Liberthson RR, Sagar K, Berkoben JP. Congenital coronary arteriovenous fistula. Report of 13 patients, review of the literature and delineation of management. Circulation. 1979;59(5):849–854. doi: 10.1161/01.cir.59.5.849. [DOI] [PubMed] [Google Scholar]
- 32.Shyam Sunder KR, Balakrishnan KG, Tharakan JA, et al. Coronary artery fistula in children and adults: a review of 25 cases with long-term observations. International Journal of Cardiology. 1997;58(1):47–53. doi: 10.1016/s0167-5273(96)02792-1. [DOI] [PubMed] [Google Scholar]
- 33.Sanders SP, Parness IA, Colan SD. Recognition of abnormal connections of coronary arteries with the use of Doppler color flow mapping. Journal of the American College of Cardiology. 1989;13(4):922–926. doi: 10.1016/0735-1097(89)90237-4. [DOI] [PubMed] [Google Scholar]
- 34.Kamiya H, Yasuda T, Nagamine H, et al. Surgical treatment of congenital coronary artery fistulas: 27 Years' experience and a review of the literature. Journal of Cardiac Surgery. 2002;17(2):173–177. doi: 10.1111/j.1540-8191.2002.tb01195.x. [DOI] [PubMed] [Google Scholar]
- 35.Cheung DLC, Au WK, Cheung HHC, Chiu CSW, Lee WT. Coronary artery fistulas: long-term results of surgical correction. Annals of Thoracic Surgery. 2001;71(1):190–195. doi: 10.1016/s0003-4975(00)01862-2. [DOI] [PubMed] [Google Scholar]
- 36.Dorros G, Thota V, Ramireddy K, Joseph G. Catheter-based techniques for closure of coronary fistulae. Catheterization and Cardiovascular Interventions. 1999;46(2):143–150. doi: 10.1002/(SICI)1522-726X(199902)46:2<143::AID-CCD6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 37.Reidy JF, Anjos RT, Qureshi SA, Baker EJ, Tynan MJ. Transcatheter embolization in the treatment of coronary artery fistulas. Journal of the American College of Cardiology. 1991;18(1):187–192. doi: 10.1016/s0735-1097(10)80239-6. [DOI] [PubMed] [Google Scholar]
- 38.Hartnell GG. Embolization in the treatment of acquired and congenital abnormalities of the heart and thorax. Radiographics. 1993;13(6):1349–1363. doi: 10.1148/radiographics.13.6.8290729. [DOI] [PubMed] [Google Scholar]
- 39.Iida R, Yamamoto T, Suzuki T, Saeki S, Ogawa S. The usefulness of intraoperative transesophageal echocardiography to identify the site of drainage of coronary artery fistula. Anesthesia and Analgesia. 2005;101(2):330–331. doi: 10.1213/01.ANE.0000158466.63486.B5. [DOI] [PubMed] [Google Scholar]
- 40.Lessick J, Kumar G, Beyar R, Lorber A, Engel A. Anomalous origin of a posterior descending artery from the right pulmonary artery: report of a rare case diagnosed by multidetector computed tomography angiography. Journal of Computer Assisted Tomography. 2004;28(6):857–859. doi: 10.1097/00004728-200411000-00023. [DOI] [PubMed] [Google Scholar]
- 41.Parga JR, Ikari NM, Bustamante LN, et al. Case report: MRI evaluation of congenital coronary artery fistulae. The British Journal of Radiology. 2004;77(918):508–511. doi: 10.1259/bjr/24835123. [DOI] [PubMed] [Google Scholar]
- 42.Sapin P, Frantz E, Jain A, Nichols TC, Dehmer GJ. Coronary artery fistula: an abnormality affecting all age groups. Medicine. 1990;69(2):101–113. [PubMed] [Google Scholar]
- 43.Hackett D, Hallidie-Smith KA. Spontaneous closure of coronary artery fistula. British Heart Journal. 1984;52(4):477–479. doi: 10.1136/hrt.52.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Umaña E, Massey CV, Painter JA. Myocardial ischemia secondary to a large coronary-pulmonary fistula. Angiology. 2002;53(3):353–357. doi: 10.1177/000331970205300315. [DOI] [PubMed] [Google Scholar]
- 45.Balanescu S, Sangiorgi G, Castelvecchio S, Medda M, Inglese L. Coronary artery fistulas: clinical consequences and methods of closure: a literature review. Italian Heart Journal. 2001;2(9):669–676. [PubMed] [Google Scholar]
- 46.Wang S, Wu Q, Hu S, et al. Surgical treatment of 52 patients with congenital coronary artery fistulas. Chinese Medical Journal. 2001;114(7):752–755. [PubMed] [Google Scholar]
- 47.Hartnell GG, Jordan SC. Balloon embolisation of a coronary arterial fistula. International Journal of Cardiology. 1990;29(3):381–383. doi: 10.1016/0167-5273(90)90129-s. [DOI] [PubMed] [Google Scholar]
- 48.Qureshi SA, Tynan M. Catheter closure of coronary artery fistulas. Journal of Interventional Cardiology. 2001;14(3):299–307. doi: 10.1111/j.1540-8183.2001.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 49.Alekyan BG, Podzolkov VP, Cárdenas CE. Transcatheter coil embolization of coronary artery fistula. Asian Cardiovascular and Thoracic Annals. 2002;10(1):47–52. doi: 10.1177/021849230201000112. [DOI] [PubMed] [Google Scholar]
- 50.Armsby LR, Keane JF, Sherwood MC, Forbess JM, Perry SB, Lock JE. Management of coronary artery fistulae: patient selection and results of transcatheter closure. Journal of the American College of Cardiology. 2002;39(6):1026–1032. doi: 10.1016/s0735-1097(02)01742-4. [DOI] [PubMed] [Google Scholar]
- 51.Mullasari AS, Umesan CV, Kumar KJ. Transcatheter closure of coronary artery to pulmonary artery fistula using covered stents. Heart. 2002;87(1):p. 60. doi: 10.1136/heart.87.1.60-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sreedharan M, Prasad G, Barooah B, Dash PK. Vortex coil embolisation of coronary artery fistula. International Journal of Cardiology. 2004;94(2-3):323–324. doi: 10.1016/j.ijcard.2003.04.031. [DOI] [PubMed] [Google Scholar]
- 53.Pettersen MD, Ammash NM, Hagler DJ, Rihal CS, Cabalka AK. Endovascular stent implantation in a coronary artery to pulmonary artery fistula in a patient with pulmonary atresia with ventricular septal defect and severe cyanosis. Catheterization and Cardiovascular Interventions. 2001;54(3):358–362. doi: 10.1002/ccd.1300. [DOI] [PubMed] [Google Scholar]


