Abstract
Small animal neuroimaging has become increasingly available to researchers, expanding the breadth of questions studied with these methods. Applying these noninvasive techniques to the open questions underlying epileptogenesis is no exception. A major advantage of small animal neuroimaging is its translational appeal. Studies can be well controlled and manipulated, examining the living brain in the animal before, during, and after the disease onset or disease treatment. The results can also be compared to data collected on human patients. Over the past decade, we and others have explored metabolic patterns in animal models of epilepsy to gain insight into the circuitry underlying development of the disease. In this paper, we provide technical details on how metabolic imaging that uses 2-deoxy-2[18F]fluoro-D-glucose (18FDG) and positron emission tomography (PET) is performed and explain the strengths and limitations of these studies. We will also highlight recent advances toward understanding epileptogenesis through small animal imaging.
1. Introduction
There is a strong foundation for using neuroimaging techniques such as PET to probe circuitry underlying symptoms of patients with epilepsy. In this paper, we will highlight basic research in this area, focusing our discussion on studies conducted using imaging on animal models.
What makes using neuroimaging techniques so advantageous? First, these methods can be harnessed to dissect circuitry differences between various classifications of epilepsy. There are currently over 40 classified types of epilepsy, including generalized, focal, continuous, and reflex seizures according to the International League against Epilepsy [1]. There are also over 27 identified epilepsy syndromes classified by a myriad of seizure types, symptoms, pathology, and etiology. PET can potentially be used in the evaluation and diagnosis of differing patient populations.
Second, for a given radiotracer, brain scans from patients can be almost directly compared to small animal neuroimaging results, which can validate existing models. Even though seizure symptoms and classifications are so diverse and animal models only partially exemplify aspects of the human condition, a rich basic science literature exists that has significantly advanced our understanding of circuitry changes underlying seizures using techniques such as electrophysiology and microscopy to measure cellular changes. PET has contributed and will continue to contribute to our understanding in these areas through its major advantage of conducting whole brain analyses. Prior research has validated the use of 2-deoxy-2[18F]fluoro-D-glucose (18FDG), a radiotracer which measures tissue metabolic activity, toward this goal. Therefore as a foundation, we and others have explored circuitry in the epileptic animal model using 18FDG-PET imaging methods [2–8]. Although the limited spatial resolution of PET does not allow for the evaluation of changes at the cellular level, this disadvantage is balanced by the ability to look at overall changes throughout brain regions related to seizure symptoms. Furthermore, newer ligand-specific radiotracers make possible a more precise examination of neurotransmitter systems [9–12].
Third, neuroimaging is considered noninvasive and thus several scans can be given to the same patient over the course of the disease, or the same animal over the time course of an experiment. Therefore, the progression from naïve to acute and then to chronic seizures can truly be studied and perhaps more importantly, new treatments can be evaluated in animal subjects over time. A patient (or animal model) that has more than one seizure over time is considered to be epileptic, and the progression of epilepsy from an initial single seizure to spontaneous recurrent seizures (SRS) is called epileptogenesis. Continuing research efforts to understand the mechanisms of epileptogenesis are required to identify new molecular targets which may help control refractory seizures. Small animal neuroimaging techniques are useful in this endeavor, offering the advantage that they allow investigators to examine the living brain before the disease is induced and over the time course of symptoms and even therapies.
In the next few sections, a technical review of 18FDG-PET is given, followed by a discussion of what we have learned and can still learn by studying human epilepsy in animal models with these strategies.
2. Technical Aspects of 18FDG-PET
Three decades have passed since imaging was introduced to diagnostic medicine. Techniques such as X-ray computed tomography (CT) and magnetic resonance imaging (MRI) have allowed practitioners to evaluate structures noninvasively within patients. The evolution of positron emission tomography (PET) radiotracers has provided the opportunity to evaluate function within specific tissues or organs. One of the most widely used tracers in PET is 18FDG, which measures glucose metabolism and has been used extensively in diagnosis of cancer and epilepsy. More recently, 18FDG-PET has shown promise in the analysis of other disorders as well, including Alzheimer's disease and depression [13, 14]. Epileptic patients show interesting metabolic signatures both in-between (interictal) and during (ictal) seizures, which have helped in diagnosis and presurgical evaluation [15, 16].
Neuroimaging techniques have advantages and limitations. In the following paragraphs, we will discuss these characteristics along with technical aspects of conducting a PET scan for those unfamiliar with this imaging method.
18FDG radiopharmaceutical production begins with conversion of 18O enriched water to the isotope 18F at a cyclotron, followed by incorporation of the fluoride isotope onto the deoxyglucose molecule to make 18FDG (Figure 1(a)) [17]. After pyrogen testing, a trace amount of 18FDG is administered to a subject, where it accumulates in metabolically active tissues or organs (Figure 1(b)). As each molecule of 18FDG undergoes beta decay, a neutron and a positron are released. When the positron collides with a nearby electron (the range of which provides an inherent resolution limitation), an annihilation event occurs producing two 511 keV gamma rays released nearly 180° from each other. The noncollinearity of the gamma rays (or slight deviation from exactly 180°) provides a second physical limitation to resolution. These are detected as coincidence events by two opposite lutetium oxyorthosilicate (LSO) crystals (or other detector material) contained in the cylinder surrounding the subject in the PET camera bed portal (Figure 1(c)). Coincidence events can also occur at random, and actual events can be missed due to scatter effects introducing artifact in the data sets. Corrections for scatter effects [18], attenuation, dead time, and detector sensitivity can potentially reduce noise.
Figure 1.
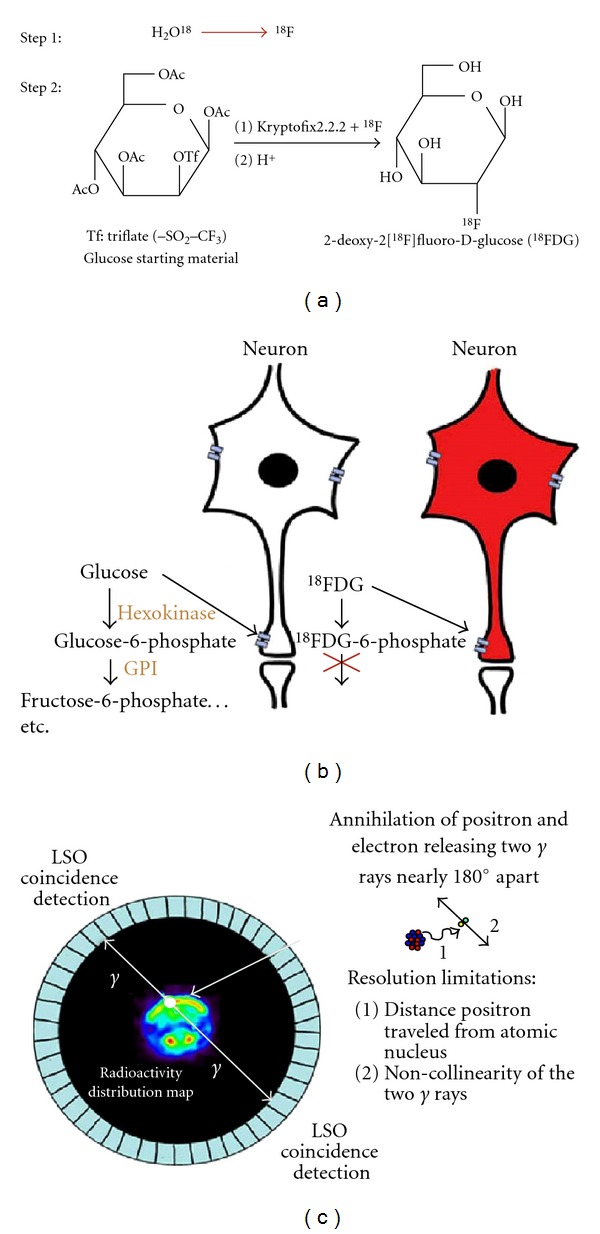
Schematic of 18FDG-PET imaging principles. (a) 18FDG production step 1: 18O enriched water (95%) is bombarded with protons in a cyclotron creating 18-flouride (18F) which is purified on a resin column and rinsed with potassium carbonate. Step 2: 18F is added with Kryptofix2.2.2 to the precursor glucose starting material and through a nucleophilic substitution, subsequent purification and removal of protecting groups, 18FDG is synthesized. (b) The radiotracer accumulates in tissue where it is transported into metabolically active cells, metabolized by hexokinase, and trapped as FDG-6-phosphate, as it is not a good substrate for further metabolism by glucose phosphate isomerase (GPI). (c) Representation of a mouse in the field of view of a small animal PET camera. When the 18F on a molecule of 18FDG undergoes beta decay, a proton turns into a neutron expelling a positron and neutrino from the atomic nucleus. The positron travels up to a few millimeters before it collides with an electron (inset). The energy of this annihilation expels 2 511 keV gamma rays (γ) approximately 180° apart from each other. These γ rays are detected simultaneously by a ring of LSO crystals that make up the PET camera.
Software programs used in conjunction with the PET camera record coincidence events and reconstruct where the decays were most likely to have occurred within the field of view. The most common reconstruction algorithm is Filtered Back Projection (FBP), which has the advantage of economical computing resources, but is restricted by noise artifacts and relatively lower spatial resolution. Expectation maximization algorithms for reconstruction of PET data were first employed over 2 decades ago [19], however at the time their demanding computing resources had restricted their clinical availability and acceptance. Many groups have developed variations on the approach reducing computational burden, decreasing time to convergence, thus allowing the algorithms to become increasingly practical for routine use [20]. Such iterative methods include ordered-subsets expectation maximization (OSEM) [21], maximum likelihood expectation maximization (MLEM) [22], and maximum a posteriori (MAP) [23]. The actual resolution depends on several factors, including the physics of the annihilation event (discussed above) and specifications of the camera used to collect the data (such as scintillation crystal performance and electronics). Data can be improved with the reconstruction methods employed but still with limitations. Typically, small animal PET scanners with a reduced ring diameter and portal size approach a spatial resolution range of 1–3 mm FWHM [24, 25], while larger human scanners can range from 4–7 mm FWHM [26].
The pharmacokinetics of 18FDG have been well studied and involve a four compartment model [27]. Normally as glucose from the circulation enters a cell via a glucose transporter (K1), it is converted into glucose-6-phosphate by the action of hexokinase during the first step of glycolysis (K3). Subsequently, glucose phosphate isomerase (GPI) converts this metabolite into fructose-6-phosphate. However, due to the substitution of a fluorine atom, the intracellular conversion of 18FDG by the enzyme hexokinase into FDG-6-phosphate yields an unsuitable substrate for further metabolism by GPI. This simplifies interpretation of results, as there are no further radioactive metabolites to complicate the data or the determination of reaction rate. Furthermore, the rate of efflux (K4) of FDG-6-phospate is very slow, trapping the radioactive molecule in the metabolically active cell. The slow efflux coupled with a half life of 109.8 minutes makes 18FDG well suited for determining metabolic rates of glucose in tissue. It also makes it ideal for studies aimed at evaluating metabolic changes from baseline concurrent with a task or behavior, including seizures. For example, increased neuronal activity during and immediately following a seizure episode would result in metabolic changes in the brain and can be visualized with 18FDG. One caveat with 18FDG is that the Harderian glands typically show a high degree of tracer uptake and can interfere with measurements made in the olfactory bulb and frontal cortex. Thus despite the limited resolution and the need for injecting a radioactive substance into the subject, the major advantage of PET is the ability to study function of the living brain over time.
3. Clinical Diagnosis and Neuroimaging Research
Early on, it was discovered that epileptic patients evaluated with 18FDG showed abnormalities in the form of hypometabolic areas in the limbic system depending on the progression of the disease. Clinicians originally hoped to use these scans to identify the region of seizure focus leading surgical resection of the aberrant region. However, it was found that the hypometabolic regions seemed to extend beyond that of the seizure focus, making it difficult to identify the exact margins. Interestingly, studies had shown that during the ictal phase, when 18FDG is administered very close to the onset of spontaneous seizures, there is an expected increase in metabolic activity in the seizure focus [28]. This of course would be helpful to a physician's strategy to extract the epileptic zone in medically intractable patients and led to development of ictal perfusion SPECT imaging with 99mTc-ethyl cysteinate dimer (ECD) or 99mTc-hexamthylpropyleneamine oxime (HMPAO) [16]. The tracer is injected into the patient during the seizure and, due to its pharmacokinetics, provides a snapshot of the metabolic activity in the brain that can be viewed later (e.g., 30–60 min for 18FDG). However, there are technical limitations to this, including coordinating the patients seizure onset with injection and that not all medical centers are equipped for this type of study [29].
4. Basic Metabolic Neuroimaging Research: Evaluating Circuitry in Animal Seizure Models
Several research groups, including ours, have employed in vivo brain imaging methods to understand changes in neurocircuitry due to acute and chronic seizures, and to study the progression of epilepsy in animal models [2, 3, 5, 7, 11, 30–37]. Furthermore, many excellent reviews have been written regarding the use and impact of imaging animal models of epilepsy [6, 29, 38–41]. This paper will highlight the important findings of recent studies.
Our initial study examined pilocarpine-induced seizures in C57Bl/6 mice [3] as a foundation for our subsequent studies in genetically modified mice [2, 30]. We confirmed that brain activity in wild-type mice can be recorded and visualized during seizures with 18FDG-PET. Specifically, we found a 33% increase in metabolic activity in the hippocampus, which correlated with the degree of seizure severity (r2 = 0.86) [3]. This result was consistent with a previous study in rats using kainate [4] and indicated that it was possible to identify regions of elevated neuronal activity during seizures. One of the benefits of 18FDG-PET, as discussed in the previous section, is that the radiotracer can be injected into an awake animal behaving normally, and become trapped in metabolically active cells. In our protocol, we inject 18FDG 10 min after pilocarpine injection to capture brain activity during the peak of the seizure behavioral response. The animals were then anesthetized 45–60 min following the 18FDG injection and a static (1 frame) 10–20 min scan was acquired.
In our next study, we developed a more detailed region of interest (ROI) and voxel-based strategy for analyzing these data. We found interesting correlations between seizures and metabolic activity in wild-type mice using Statistical Parametric Mapping (SPM), an image analysis tool to explore changes throughout the brain without predefining regions of interest. Specifically, we found positive correlations with seizure activity in the hippocampus, septum, thalamus, midbrain, olfactory bulb, and cerebellum [2]. These regional metabolic changes may represent the effect of seizure spreading: while the hippocampus, septum, and thalamus are initially required for seizure generation (as they express muscarinic (M1) acetylcholine receptors—the sites of action of pilocarpine), elevated activity is propagated downstream in additional regions likely through glutamatergic receptor mechanisms.
The neurocircuitry of the rat lithium-pilocarpine model has also been explored using neuroimaging methods [5, 7, 31]. The studies by Goffin et al. and Guo et al., [5, 7] examined changes in glucose metabolism over time using 18FDG-PET. Goffin and colleagues compared rats that had received saline or pilocarpine at three time points, baseline, day 3 following pilocarpine injection in the early silent phase of epileptogenesis and at the end of the monitoring period during the chronic phase (day 70). They found that at day 3, metabolism in most of the brain (except the cerebellum) was decreased with the biggest changes occurring in the hippocampus, entorhinal cortex, and thalamus as compared to baseline and saline treated [5]. Guo and colleagues found similar patterns conducting brain scans at day 2 (early), day 7 (latent), and day 42 (chronic) phases after initial SE induction. This group showed in the early phase, there was a significant decrease in metabolism in the entorhinal cortex [7]. Interestingly, these results paralleled our data, suggesting that hippocampal regions, which exhibit overwhelming activity during seizure induction, undergo significant changes during the latent period and result in regional hypometabolism.
In the chronic period, Goffen et al. found increased activity bilaterally in the cerebellum, pons, and medulla oblongata, along with decreased activity in the left striatum and entorhinal cortex, when comparing the seizure group to controls. Unfortunately, the method was not sensitive enough to detect changes between animals experiencing SRS and not, as there were only 3 and 4 animals in these groups to compare, respectively. This is understandable, however, since it is relatively difficult to obtain sufficient numbers of surviving animals showing SRS in the chronic phase. Therefore, the study conducted by Guo et al. employed more animals to explore chronic effects. They observed that global hypometabolism persisted through the latent phase but recovered in the chronic phase, except for the hippocampus and thalamus, which remained below baseline in animals that had shown SRS. Throughout the experiment cerebellar metabolism was not changed. Interestingly, severe entorhinal cortex hypometabolism in the early phase correlated with epileptogenesis behaviorally such that a shorter latent phase and higher frequency of SRS correlated with the degree of hypometabolism. These data provide further evidence that reduced neuronal activity in the entorhinal cortex in the early phase, and persistent hypometabolism in the hippocampus and thalamus following SRS, may underlie the progression of epileptogeneisis.
Data from our group also suggested an important role for the dorsolateral entorhinal cortex (as well as the amygdaloid nucleus, piriform cortex, and anterior striatum) during the early (silent) period, as these areas were hypometabolic in wild-type mice 7 days after pilocarpine [30]. We also found increased activity bilaterally in the thalamus, superior colliculus, and cerebellum. However, in our study the mice injected did not experience severe seizures during the induction period (average score based on the above described scale was only 1.88 ± 0.19). This finding highlights an important point that studies should address the differences between drug effects (pilocarpine/chemoconvulsant) alone versus the effect of seizure symptoms. It may be that small animal imaging is not sensitive enough to detect changes between mice experiencing SRS and not (due to intersubject variability and low resolution), however, further studies with larger groups of animals suitable for statistical analysis, either with region of interest (ROI) or whole brain voxel-based (Statistical Parametric Mapping, SPM) methods, are needed to conclusively answer this question. One recent study aimed to explore this issue by Navarro Mora and colleagues, found that there are indeed neurocircuitry changes in rats injected with pilocarpine, but they did not develop SE [34]. In other words, animals that did not experience severe seizures immediately following pilocarpine injection could still exhibit SRS approximately 8 months later, showing a long latency period resembling human pathology.
Transgenic animals are more commonly being used in PET studies, and we have conducted two studies so far. One of our studies aimed to evaluate the impact of knocking out tissue-plasminogen activator (tPA) on neuronal circuitry during seizures. tPA is a serine protease that converts the zymogen plasminogen (plg) to the active protease plasmin [42] and initiates a potent proteolytic cascade with a well-characterized function in thrombolysis. Interestingly, studies from our laboratory have shown that tPA may be a molecular mediator of seizures in animal models, and thus a potential pharmacotherapeutic target, therefore we studied seizure propagation in wild-type versus tPA−/− mice using 18FDG-PET. Our results were consistent with previous studies, showing that tPA was necessary for facilitating pilocarpine-induced seizures: the seizure symptoms in the knockout animals did not exceed partial body clonus (stage 3) [2]. We conducted both ROI (regional) and SPM (voxel-based) analyses to evaluate potential effects on metabolic activity between the two genotypes. Although pilocarpine injection did significantly increase activity in the limbic system and several additional brain areas, we did not observe significant differences between genotypes with the ROI method, except in the olfactory bulb, which was active in the knockout animals. Therefore we employed a more sensitive voxel-based analysis method, which created a visual map of brain areas significantly increased in conjunction with seizure activity (via a covariate analysis). This analysis revealed clusters of activation in the hippocampus, basal forebrain and septum, and thalamus that were more widespread in wild-type mice compared to the knockout animals, consistent with the behavioral data. Interestingly, midbrain, cerebellum, and olfactory bulb increases were also unique to knockout mice. Overall, these data suggested the lack of seizure spreading mechanisms instead of reduced excitatory potential in the absence of tPA.
Secondly, we explored whether changes in glial function were a component of metabolic changes in mice following pilocarpine-induced seizures [30]. We hypothesized that such changes could parallel metabolic abnormalities observed in epileptic patients [43, 44]. We observed differences in seizure severity when unilaterally ablating microglia in the hippocampus. Our goal using PET was also to find the brain regions involved in this behavioral divergence. We found increased 18FDG uptake during seizures in the septum, thalamus, hippocampus, midbrain, and cerebellum, along with decreases in the striatum which were consistent with our previous findings [2]. However, we did see reduced metabolic activation in the ipsilateral hippocampus corresponding to the location of microglia ablation. While we interpreted this result with caution, it is possible that activated microglia may contribute a small proportion of the metabolic signal.
5. Beyond Metabolic Imaging: Histology, MRI, and Radioligands
An important factor in interpreting small animal imaging data is the relatively low spatial resolution compared to more traditional imaging methods, such as microscopy. However, when combined with histological methods the potential amount of information that we can derive is increased. Interestingly, one study has already explored the issue of the hypometabolic hippocampus and degree of hippocampal sclerosis [45]. Hippocampal damage was quantified with quantitative magnetic resonance imaging (QMRI) and samples of human tissue, epileptic foci removed via surgical resection, were analyzed histopathologically (neuronal loss, proliferation of glial cells, and mossy fiber sprouting). The severity of metabolic hypometabolism did not seem to correlate with hippocampal damage in these patients, thus suggesting that there may not be a direct correlation between hippocampal sclerosis and FDG imaging data. However in another study, FDG hypometabolism was significantly correlated with degree of hippocampal atrophy [12].
Future studies will continue to explore the neurocircuitry of epileptogenesis by assessing different neurotransmitter systems with radiotracer ligands to various receptors and molecular markers. Animal models are particularly suited for studying potentially newly developed radiotracer ligands that may be markers for epileptogenesis before they are explored in human patients. Several well-established radiotracers have already been examined in both human and animal models. These include the 5HT1A serotonin receptor ligand 18F-FCWAY [10], dopamine D2 and D3 receptor ligand 18F-Fallypride [12, 32], and GABA(A) density with 11C-Flumazenil [33]. Liew et al. found that 18F-FCWAY binding was reduced in the mesial temporal region of epileptic patients despite normal MRI. They suggested that 18F-FCWAY may be a more accurate measure than FDG, since FDG could not distinguish mesial from lateral temporal foci in their small patient sample [10]. Yakushev et al. showed that D2/3 receptor availability appeared to be decreased by 27% bilaterally in the anterior striatum of pilocarpine-treated rats, a finding that could be interpreted as an increase in dopamine in the synapse [32]. This result may explain why we also observed a decrease in metabolic activity in the same region of pilocarpine-treated mice [30] and may suggest that increased dopamine tonus is activated at the stage of generalization of limbic seizures to exert an inhibitory restraint on seizure propagation [32]. Werhahn and colleagues also showed reduced 18F-Fallypride in the borders of epileptic foci in patients with mesial TLE and hippocampal sclerosis, warranting further clinical evaluation [12]. The study by Liefaard et al. reported that GABA(A) receptor density was decreased by 36% in the cortex of kindled rats. They suggest that this decrease is a potential mechanism underlying pharmacoresistance to midazolam [33]. Overall, these studies provide an interesting framework for future experimentation.
Although only a few studies have explored various therapeutic strategies for epilepsy in small animals thus far [11, 35, 46], experiments toward this end are likely to increase in the near future. As suggested above by the Liefaard study, examining the receptors targeted by drugs can be an interesting method to explore therapeutic effects and pharmacoresistance. The cannabinoid system was also studied using 18F-MK-9470 to examine the effect of chronic treatment with valproate (VPA) and levetiracetam (LEV) [11]. This study showed that VPA, but not LEV, globally increased cerebral binding of the radiotracer suggesting an indirect effect for VPA leading to the upregulation of CB1 receptors and a potential new mechanism for its efficacy. Vagus nerve stimulation was also explored using glucose metabolism, which revealed decreased activity in the hippocampus with increases bilaterally in the olfactory bulbs and disruption in striatal activity [46]. Interestingly novel applications of deep brain stimulation (DBS) to different areas for various neurological and mental disorders are being evaluated both clinically and preclinically. Small animal imaging also provides an avenue to explore neurocircuitry changes throughout the brain as a result of DBS, as shown by Gao and colleagues. DBS to the anterior thalamic nucleus (ATN) and lesion to the ATN were evaluated in this study showing these strategies may improve symptoms by different methods. This target may be clinically relevant for epilepsy based on its connectivity to the cortex and limbic structures. ATN-DBS in naïve rats resulted in elevated metabolic activity in the target region, in the hippocampus and thalamus, and decreased activity in the frontal cortex and cingulated gyrus, while ATN-lesion only reduced activity in the ATN [35]. Importantly, metabolic imaging showed that the DBS changes were both excitatory and inhibitory and also reversible, unlike those of lesion therapies, and suggested that the hippocampus and thalamus may be engaged during DBS to ameliorate seizure spreading.
In addition to PET, functional magnetic resonance imaging (fMRI) can be useful for measuring neuronal activity changes during epileptogenesis. This imaging modality indirectly measures neuronal activity based on the hemodynamic response, the fact that more neuronal activity requires more oxygen to be delivered through the blood stream. The signal measured is referred to as the BOLD signal (meaning Blood-Oxygen-Level Dependence), where changes in the ratio of paramagnetic deoxyhemoglobin and diamagnetic oxyhemoglobin are measured with the MRI scanner. A review by Blumenfeld elegantly describes these methods and how they can be applied to epilepsy research [47], and a study by the same author and Engolt et al., showed interesting results [48]. The study explored the differences in brain waves (via electroencephalography (EEG)) and blood flow (fMRI) between behaviorally mild partial seizures and those which propagate and become generalized convulsive seizures. The authors found that mild seizures increased the BOLD signal (and flow related cerebral blood volume, CBV, after injection of an exogenous paramagnetic contrast) in the hippocampus, thalamus, and septum, while the signal decreased in the orbitofrontal, anterior cingulate, and retrosplenial cortex, corresponding to slow waves in the frontal cortex. However during propagated seizures, the signal to all of these regions was increased and included other areas of the primary somatosensory cortex, corresponding with fast polyspike activity. This study suggests a mechanism underlying the cognitive impairments experienced by patients exhibiting mild partial seizures, and using several imaging methods, it begins to unravel neurocircuitry patterns at the various stages of epilepsy.
6. Summary
The studies reviewed here, both from other researchers and including our own, show that despite some limitations, small animal imaging is an extremely useful technique for understanding circuitry changes in the brain during epileptogenesis. Future studies will continue to investigate the metabolic effects of various therapeutic modalities in animal models. As well, targeted cellular changes during seizure symptoms and treatments will continue to be explored through more specific radioligands. Furthermore, correlating circuitry changes with end point histology in animal models will expand our understanding of how cellular changes relate to global metabolic dysfunction in epileptic patients.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) with funding to S. E. T. (R01NS042168). The authors are grateful to Joanna Fowler and David Alexoff for helpful discussions and Nora Ruth for editing. We would also like to thank Fritz Henn for support to M. M. M while writing this manuscript.
References
- 1.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE commission on classification and terminology, 2005–2009. Epilepsia. 2010;51(4):676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 2.Mirrione MM, Schiffer WK, Fowler JS, Alexoff DL, Dewey SL, Tsirka SE. A novel approach for imaging brain-behavior relationships in mice reveals unexpected metabolic patterns during seizures in the absence of tissue plasminogen activator. NeuroImage. 2007;38(1):34–42. doi: 10.1016/j.neuroimage.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirrione MM, Schiffer WK, Siddiq M, Dewey SL, Tsirka SE. PET imaging of glucose metabolism in a mouse model of temporal lobe epilepsy. Synapse. 2006;59(2):119–121. doi: 10.1002/syn.20216. [DOI] [PubMed] [Google Scholar]
- 4.Kornblum HI, Araujo DM, Annala AJ, Tatsukawa KJ, Phelps ME, Cherry SR. In vivo imaging of neuronal activation and plasticity in the rat brain by high resolution positron emission tomography (microPET) Nature Biotechnology. 2000;18(6):655–660. doi: 10.1038/76509. [DOI] [PubMed] [Google Scholar]
- 5.Goffin K, Paesschen WV, Dupont P, Laere KV. Longitudinal microPET imaging of brain glucose metabolism in rat lithium-pilocarpine model of epilepsy. Experimental Neurology. 2009;217(1):205–209. doi: 10.1016/j.expneurol.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien TJ, Jupp B. In-vivo imaging with small animal FDG-PET: a tool to unlock the secrets of epileptogenesis? Experimental Neurology. 2009;220(1):1–4. doi: 10.1016/j.expneurol.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Guo Y, Gao F, Wang S, et al. In vivo mapping of temporospatial changes in glucose utilization in rat brain during epileptogenesis: an 18F-fluorodeoxyglucose-small animal positron emission tomography study. Neuroscience. 2009;162(4):972–979. doi: 10.1016/j.neuroscience.2009.05.041. [DOI] [PubMed] [Google Scholar]
- 8.Mirrione MM, Konomos DK, Gravanis I, et al. Microglial ablation and lipopolysaccharide preconditioning affects pilocarpine-induced seizures in mice. Neurobiology of Disease. 2010;39(1):85–97. doi: 10.1016/j.nbd.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assem-Hilger E, et al. Central serotonin 1A receptor binding in temporal lobe epilepsy: a [carbonyl-(11)C]WAY-100635 PET study. Epilepsy and Behavior. 2010;19(3):467–473. doi: 10.1016/j.yebeh.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 10.Liew CJ, et al. 18F-FCWAY and 18F-FDG PET in MRI-negative temporal lobe epilepsy. Epilepsia. 2009;50(2):234–239. doi: 10.1111/j.1528-1167.2008.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goffin K, Bormans G, Casteels C, et al. An in vivo [18F]MK-9470 microPET study of type 1 cannabinoid receptor binding in Wistar rats after chronic administration of valproate and levetiracetam. Neuropharmacology. 2008;54(7):1103–1106. doi: 10.1016/j.neuropharm.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 12.Werhahn KJ, Landvogt C, Klimpe S, et al. Decreased dopamine D2/D3-receptor binding in temporal lobe epilepsy: an [18F]Fallypride PET study. Epilepsia. 2006;47(8):1392–1396. doi: 10.1111/j.1528-1167.2006.00561.x. [DOI] [PubMed] [Google Scholar]
- 13.Bailey RL, et al. Dietary supplement use in the United States, 2003–2006. Journal of Nutrition. 2011;141(2):261–266. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joosse A, et al. Gender differences in melanoma survival: female patients have a decreased risk of metastasis. Journal of Investigative Dermatology. 2011;131(3):719–726. doi: 10.1038/jid.2010.354. [DOI] [PubMed] [Google Scholar]
- 15.Fong CY, Delgado-Escueta AV. Ictal PET in temporal lobe epilepsy. Journal of Neurology, Neurosurgery and Psychiatry. 1999;67(3):p. 409. doi: 10.1136/jnnp.67.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kazemi NJ, Worrell GA, Stead SM, et al. Ictal SPECT statistical parametric mapping in temporal lobe epilepsy surgery. Neurology. 2010;74(1):70–76. doi: 10.1212/WNL.0b013e3181c7da20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowler JS, Wolf AP. 2-deoxy-2-[18F]fluoro-d-glucose for metabolic studies: current status. International Journal of Radiation Applications and Instrumentation Part A. 1986;37(8):663–668. doi: 10.1016/0883-2889(86)90259-5. [DOI] [PubMed] [Google Scholar]
- 18.Alexoff DL, Vaska P, Marsteller D, et al. Reproducibility of 11C-raclopride binding in the rat brain measured with the microPET R4: effects of scatter correction and tracer specific activity. Journal of Nuclear Medicine. 2003;44(5):815–822. [PubMed] [Google Scholar]
- 19.Shepp LA, Vardi Y. Maximum likelihood reconstruction for emission tomography. IEEE Transactions on Medical Imaging. 1982;1(2):113–122. doi: 10.1109/TMI.1982.4307558. [DOI] [PubMed] [Google Scholar]
- 20.Qi J, Leahy RM. Iterative reconstruction techniques in emission computed tomography. Physics in Medicine and Biology. 2006;51(15):R541–R578. doi: 10.1088/0031-9155/51/15/R01. [DOI] [PubMed] [Google Scholar]
- 21.Hudson HM, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Transactions on Medical Imaging. 1994;13(4):601–609. doi: 10.1109/42.363108. [DOI] [PubMed] [Google Scholar]
- 22.Nuyts J, Michel C, Dupont P. Maximum-likelihood expectation-maximization reconstruction of sinograms with arbitrary noise distribution using NEC-transformations. IEEE Transactions on Medical Imaging. 2001;20(5):365–375. doi: 10.1109/42.925290. [DOI] [PubMed] [Google Scholar]
- 23.Bai B, Li Q, Holdsworth CH, et al. Model-based normalization for iterative 3D PET image reconstruction. Physics in Medicine and Biology. 2002;47(15):2773–2784. doi: 10.1088/0031-9155/47/15/316. [DOI] [PubMed] [Google Scholar]
- 24.Knoess C, Siegel S, Smith A, et al. Performance evaluation of the microPET R4 PET scanner for rodents. European Journal of Nuclear Medicine and Molecular Imaging. 2003;30(5):737–747. doi: 10.1007/s00259-002-1052-6. [DOI] [PubMed] [Google Scholar]
- 25.Tai YC, Ruangma A, Rowland D, et al. Performance evaluation of the microPET focus: a third-generation microPET scanner dedicated to animal imaging. Journal of Nuclear Medicine. 2005;46(3):455–463. [PubMed] [Google Scholar]
- 26.Tarantola G, Zito F, Gerundini P. PET instrumentation and reconstruction algorithms in whole-body applications. Journal of Nuclear Medicine. 2003;44(5):756–769. [PubMed] [Google Scholar]
- 27.Phelps ME, Huang SC, Hoffman EJ. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Annals of Neurology. 1979;6(5):371–388. doi: 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]
- 28.Millan E, Abou-Khalil B, Delbeke D, Konrad P. Frontal localization of absence seizures demonstrated by ictal positron emission tomography. Epilepsy and Behavior. 2001;2(1):54–60. doi: 10.1006/ebeh.2001.0147. [DOI] [PubMed] [Google Scholar]
- 29.Goffin K, Dedeurwaerdere S, Van Laere K, Van Paesschen W. Neuronuclear assessment of patients with epilepsy. Seminars in Nuclear Medicine. 2008;38(4):227–239. doi: 10.1053/j.semnuclmed.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Nobre GP, et al. Coupled-channel calculation of nonelastic cross sections using a density-functional structure model. Physical Review Letters. 2010;105(20) doi: 10.1103/PhysRevLett.105.202502. Article ID 202502. [DOI] [PubMed] [Google Scholar]
- 31.Sroczynski G, et al. Decision-analytic modeling to evaluate the long-term effectiveness and cost-effectiveness of HPV-DNA testing in primary cervical cancer screening in Germany. GMS Health Technology Assessment. 2010;6, article Doc05 doi: 10.3205/hta000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yakushev IY, Dupont E, Buchholz HG, et al. In vivo imaging of dopamine receptors in a model of temporal lobe epilepsy. Epilepsia. 2010;51(3):415–422. doi: 10.1111/j.1528-1167.2009.02272.x. [DOI] [PubMed] [Google Scholar]
- 33.Liefaard LC, Ploeger BA, Molthoff CFM, et al. Changes in GABAA receptor properties in amygdala kindled animals: in vivo studies using [11C]flumazenil and positron emission tomography. Epilepsia. 2009;50(1):88–98. doi: 10.1111/j.1528-1167.2008.01763.x. [DOI] [PubMed] [Google Scholar]
- 34.Navarro Mora G, Bramanti P, Osculati F, et al. Does pilocarpine-induced epilepsy in adult rats require status epilepticus? PloS one. 2009;4, article e5759(6) doi: 10.1371/journal.pone.0005759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao F, Guo Y, Zhang H, et al. Anterior thalamic nucleus stimulation modulates regional cerebral metabolism: an FDG-MicroPET study in rats. Neurobiology of Disease. 2009;34(3):477–483. doi: 10.1016/j.nbd.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Jupp B, Williams JP, Tesiram YA, Vosmansky M, O’Brien TJ. MRI compatible electrodes for the induction of amygdala kindling in rats. Journal of Neuroscience Methods. 2006;155(1):72–76. doi: 10.1016/j.jneumeth.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 37.Jupp B, Williams JP, Tesiram YA, Vosmansky M, O’Brien TJ. Hippocampal T2 signal change during amygdala kindling epileptogenesis. Epilepsia. 2006;47(1):41–46. doi: 10.1111/j.1528-1167.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- 38.Dedeurwaerdere S, Jupp B, O’Brien TJ. Positron emission tomography in basic epilepsy research: a view of the epileptic brain. Epilepsia. 2007;48, supplement 4:56–64. doi: 10.1111/j.1528-1167.2007.01242.x. [DOI] [PubMed] [Google Scholar]
- 39.Duncan J. The current status of neuroimaging for epilepsy. Current Opinion in Neurology. 2009;22(2):179–184. doi: 10.1097/WCO.0b013e328328f260. [DOI] [PubMed] [Google Scholar]
- 40.Duncan JS. Neuroimaging methods to evaluate the etiology and consequences of epilepsy. Epilepsy Research. 2002;50(1-2):131–140. doi: 10.1016/s0920-1211(02)00075-x. [DOI] [PubMed] [Google Scholar]
- 41.Jupp B, O’Brien TJ. Application of coregistration for imaging of animal models of epilepsy. Epilepsia. 2007;48, supplement 4:82–89. doi: 10.1111/j.1528-1167.2007.01245.x. [DOI] [PubMed] [Google Scholar]
- 42.Vassalli JD, Sappino AP, Belin D. The plasminogen activator/plasmin system. Journal of Clinical Investigation. 1991;88(4):1067–1072. doi: 10.1172/JCI115405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altay EE, Fessler AJ, Gallagher M, et al. Correlation of severity of FDG-PET hypometabolism and interictal regional delta slowing in temporal lobe epilepsy. Epilepsia. 2005;46(4):573–576. doi: 10.1111/j.0013-9580.2005.08204.x. [DOI] [PubMed] [Google Scholar]
- 44.Vielhaber S, Von Oertzen JH, Kudin AF, et al. Correlation of hippocampal glucose oxidation capacity and interictal FDG-PET in temporal lobe epilepsy. Epilepsia. 2003;44(2):193–199. doi: 10.1046/j.1528-1157.2003.38102.x. [DOI] [PubMed] [Google Scholar]
- 45.Lamusuo S, Jutila L, Ylinen A, et al. [18F]FDG-PET reveals temporal hypometabolism in patients with temporal lobe epilepsy even when quantitative MRI and histopathological analysis show only mild hippocampal damage. Archives of Neurology. 2001;58(6):933–939. doi: 10.1001/archneur.58.6.933. [DOI] [PubMed] [Google Scholar]
- 46.Dedeurwaerdere S, Cornelissen B, Van Laere K, et al. Small animal positron emission tomography during vagus nerve stimulation in rats: a pilot study. Epilepsy Research. 2005;67(3):133–141. doi: 10.1016/j.eplepsyres.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Blumenfeld H. Functional MRI studies of animal models in epilepsy. Epilepsia. 2007;48, supplement 4:18–26. doi: 10.1111/j.1528-1167.2007.01238.x. [DOI] [PubMed] [Google Scholar]
- 48.Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H. Remote effects of focal hippocampal seizures on the rat neocortex. Journal of Neuroscience. 2008;28(36):9066–9081. doi: 10.1523/JNEUROSCI.2014-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


