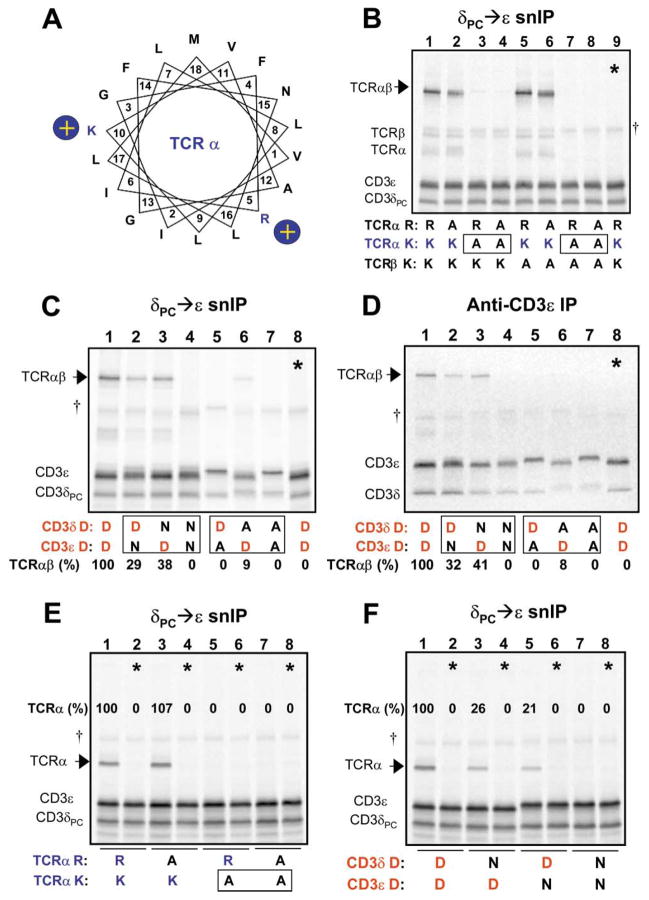
Figure 2. Interaction Among Three TM Domains that Requires One Basic and Two Acidic TM Residues.
(A) A helical wheel diagram shows the relative positions of the two basic residues in the TCRα TM region. Position 1 (V, valine) represents the first predicted TM residue at the extracellular (or ER luminal) face of the membrane. A series of experiments was performed to examine the requirements for basic and acidic TM residues in the association of CD3δε with TCR.
(B–D) analyze the four-chain TCRαβ-CD3δε complex, and (E and F) demonstrate that the same polar residues are relevant for the three-chain TCRα-CD3δε complex. CD3 heterodimers were isolated by CD3δPC→CD3ε snIP (B, C, E, and F) or single anti-CD3ε IP (D) (IP strategy indicated above each image), in order to determine the effect of mutations on assembly with TCR (arrows). In (B), the basic TM residues in the TCR chains were substituted in all possible combinations to assess their role in assembly with CD3δε (R-arginine, K-lysine, and A-alanine). The TCRα TM lysine is necessary and sufficient for this assembly step, as TCR association was lost only when this residue was substituted (boxes). The same result was obtained when TCRβ was omitted in the assembly reaction (E).
(C and D) Both acidic TM residues in CD3δε are required for association with TCR. While substitution of either aspartic acid residue with alanine [D→A] severely reduced or eliminated TCR association, conservative substitution with asparagine [D→N] was tolerated to a significant degree in either CD3 chain, but not both.
(D) The same result was obtained regardless of whether an epitope tag was used in the IP (C) or untagged complexes were precipitated with a mAb to the CD3ε extracellular domain (D).
Control lanes (*) consisted of TCR and CD3 components assembled in separate reactions and mixed prior to solubilization. Quantitation of associated TCR proteins was done by densitometry using a phosphor imager, and values are expressed as percentages relative to levels of association observed among wild-type proteins for each experiment.