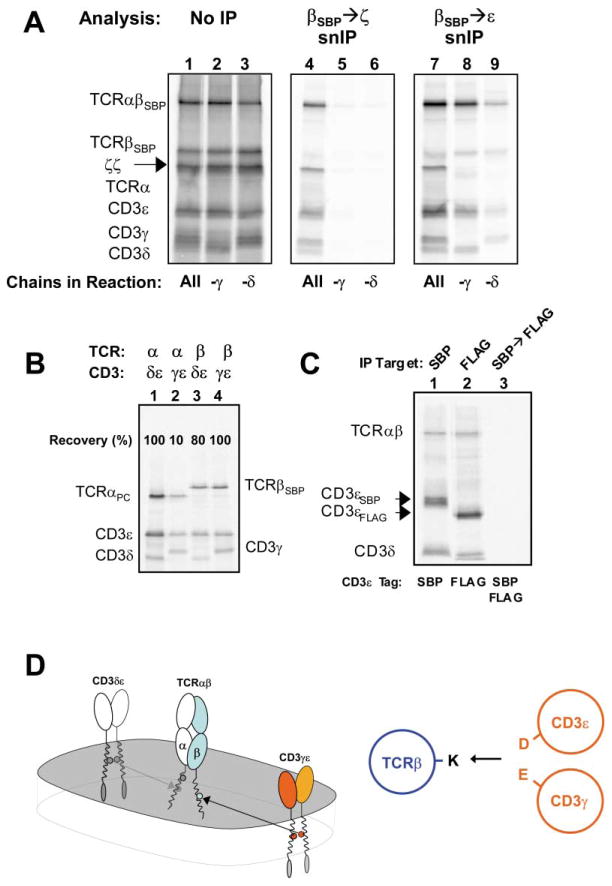
Figure 5. Evidence that TCRα-CD3δε and TCRβ-CD3γε Interactions Are Similar in the Membrane.
(A) Both CD3γ and δ are required for ζ-chain association. Reactions contained all TCR-CD3 components, as indicated below gels (lanes 1, 4, and 7), or singly lacked γ (lanes 2, 5, and 8), or δ (lanes 3, 6, and 9). No fully assembled complexes were recovered from reactions lacking either CD3γ or CD3δ (lanes 5 and 6; middle image), but TCR-CD3 sub-complexes not containing ζ chain were revealed by alternative snIP analysis of the same reactions (right image). Loading controls are provided as for previous experiments (left image).
(B) TCRβ can associate with either CD3 heterodimer in three-chain assembly reactions. TCRαPC or βSBP were each cotranslated with CD3δε or γε and three-chain complexes were isolated by αPC→ε (lanes 1 and 2) or βSBP→ε (lanes 3 and 4) snIP.
(C) TCRαβ-CD3δε complexes contain only one CD3δε heterodimer. A strategy similar to that employed in Figures 1E and 1F was used to assess whether two CD3δε dimers could associate with a single TCR.
(D) CD3γε associates with TCR via the TCRβ TM lysine (K) in a TM helical interaction similar to that seen for TCRδ-CD3δε. Two views are provided as in Figure 3E.