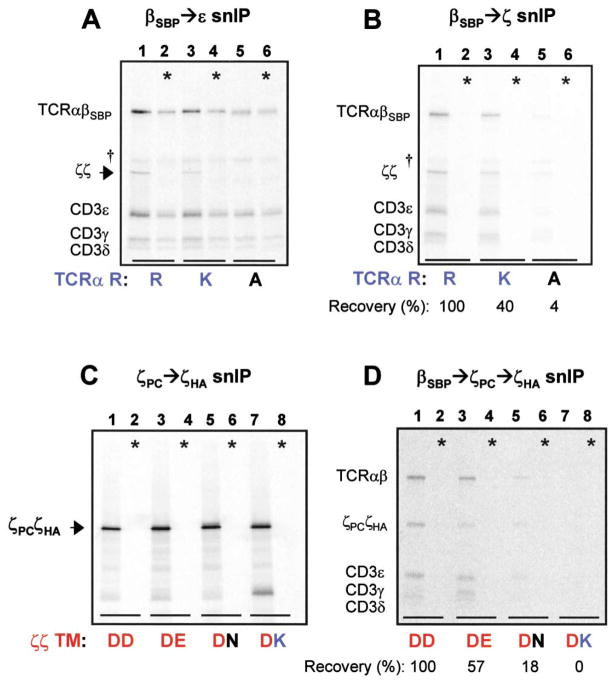
Figure 6. The TCRα TM Arginine and Both ζ Chain TM Aspartic Acid Residues Are Important for ζζ Association.
(A and B) TCRα TM arginine is important for ζ chain association. Full assembly reactions were analyzed using two different snIP strategies to examine effects of conservative and non-conservative substitutions.
(A) Specific loss of ζζ homodimer (arrowhead) was observed when TCRα TM arginine was mutated to alanine [R→A] (lane 5).
(B) Quantitation of fully assembled complexes revealed that the R→K substitution reduced ζζ association to less than half of wild-type levels (lane 3), while the R→A substitution reduced ζζ association to almost undetectable levels (lane 5). In control reactions (*), TCR-CD3 components and ζζ homodimers were assembled separately and mixed prior to solubilization.
(C and D) Both ζ chain TM aspartic acid residues are important for ζζ association.
(C) Disulfide-linked ζζ mixed dimers containing two different ζ chains in each reaction were isolated by ζPC→ζHA snIP and separated by non-reducing SDS-PAGE. Symbols indicate the wild-type dimer (DD), and mixed dimers in which the aspartic acid of one ζ chain was substituted with glutamic acid (DE), asparagine (DN), or lysine (DK). All combinations efficiently formed covalent mixed dimers. Application of this approach to full assembly reactions (D) revealed reduced ζζ assembly with TCR when one ζ chain carried a conservative substitution (DE, lane 3; DN, lane 5), and elimination of the interaction by a non-conservative substitution (DK, lane 7). In control reactions (*), full complexes with each ζ chain were assembled separately and mixed prior to solubilization.