Abstract
The purpose of this article is to report a case of unusual oral contraceptive induced periodontal endocrinopathies and its treatment approach. A 32-year-old female patient, having fair oral hygiene, presented with painless, soft, diffuse gingival enlargement on severely compromised periodontium (⩾ 4 mm generalized alveolar bone loss and attachment loss). The patient had on oral contraceptive over five years that was discontinued six months ago. When the lesion was not reversed following withdrawal of the pill or by repeated non-surgical measures, biopsy was performed to establish the diagnosis that revealed microscopic findings similar to that seen in gingival enlargement in pregnancy. Pocket depth, tooth mobility and gingival enlargement were reduced remarkably following periodontal surgery. After surgical intervention, close follow-up for over three years revealed no evidence of recurrence of gingival enlargement and progressive attachment loss. We conclude that periodontal surgery, patient compliance and comprehensive maintenance care are effective to return healthy periodontal status in such conditions.
Keywords: Oral contraceptive, gingival enlargement, non-surgical measure, biopsy, attachment loss, periodontal surgery, comprehensive maintenance care
INTRODUCTION
The role of steroid hormones in the pathogenesis of periodontal diseases is well established. Oral contraceptives (OCs) may enhance periodontal breakdown by reducing the resistance to dental plaque and may induce gingival enlargement (GE) in otherwise healthy females.1,2 OCs accentuate the gingival response to local irritants similar to that seen in pregnancy.3 The incidence and severity of gingival diseases are positively correlated with plasma sex hormone concentrations and duration of use.4 The long term use of OC may cause increased gingival inflammation, clinical attachment loss (CAL) and gingival enlargement.2,4,5 In contrast, a clinical study was unable to demonstrate any effect of low dose OCs on gingival tissue.6 Human gingiva contains estrogen and progesterone receptors. The latter influence the periodontal tissues to act as target organ for sex hormones.2 In most cases, the GE was reversed when OC was discontinued or the dosage reduced.2 We report here a case of successfully treated OC induced periodontal endocrinopathies (OCIPE).
CASE REPORT
In May 2007, a 32-year-old, otherwise healthy woman reported in our Periodontal clinic, with chief complaint of gum bleeding followed by swelling of gingiva for the last four years. The condition was first noticed by the patient after two years of combined pill (Lynestrenol 2.5 mg plus Ethinyl oestradiol 50 μg - Sukhi, Famy Care Ltd, India) intake whereas tooth mobility appeared after 3.5 years. She underwent Phase-I therapy (scaling & root planning-SRP) twice by her dentist following withdrawal of OC six months ago that resulted in improvement of bleeding tendency only. The estimated value of plasma estradiol and progesterone level at the stage of OC use was 81pg/ml and 16ng/ml respectively. The plasma estradiol and progesterone level was decreased to 75pg/ml and 12ng/ml respectively after withdrawal of OC. Family history of GE, other drug histories and menstrual history were non-contributory. Periodontal examination revealed painless, reddish-pink, soft, diffuse GE over severely compromised periodontium, mild to severe generalized CAL in spite of fair oral hygiene, insufficient attached gingiva (AG) in second and third quadrant along with multiple mobile (Miller’s Degree 2) teeth. The recorded pocket depth and tooth mobility of diseased periodontium is shown in Table 1.
Table 1.
Pocket depth and tooth mobility of diseased periodontium.
| Pocket Depth (Mean±SD) | Tooth Mobility | |||
|---|---|---|---|---|
| Pre-Operative (mm) | Post-Operative (mm) | Pre-Operative (Miller’s Class) | Post-Operative (Miller’s Class) | |
| Quadrant-1 | 4.33±0.58 | 1.97±0.58 | Degree 2 | Degree 0 |
| Quadrant-2 | 4.75±0.52 | 2.10±0.38 | Degree 2 | Degree 1 |
| Quadrant-3 | 5.10±0.42 | 1.37±0.42 | Degree 2 | Degree 1 |
| Quadrant-4 | 4.08±0.65 | 2.12±0.22 | Degree 2 | Degree 1 |
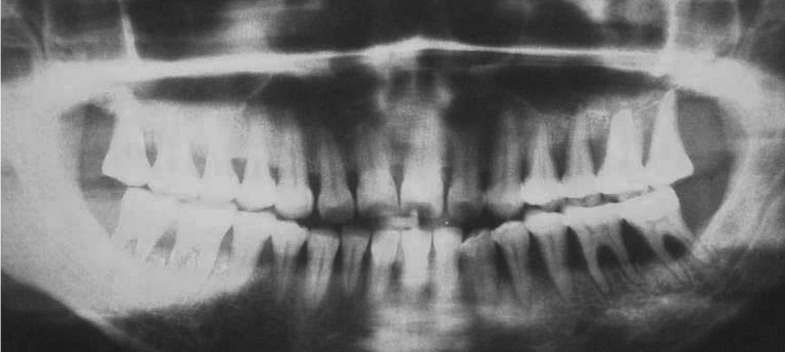
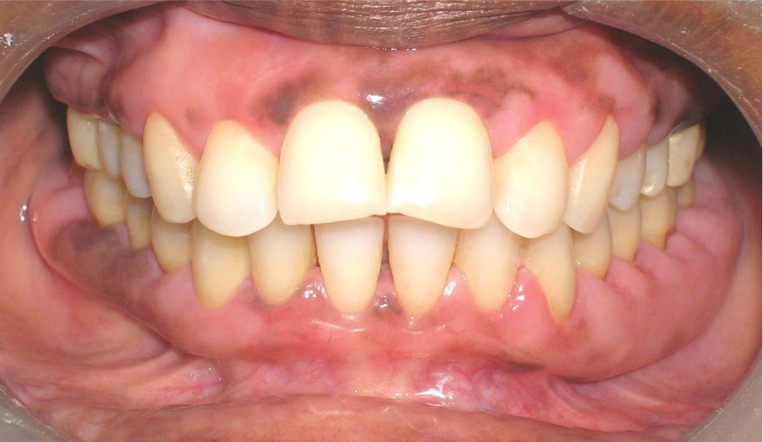
After thorough history taking and clinical examination, the patient was advised to do necessary blood examinations and orthopantomogram (OPG) (Figure 1). Patient education, motivation and oral hygiene instructions (OHIs) were explained to her. Considering patient’s history, oral examination and evaluating the reports of investigations (i.e., normal blood picture, normal plasma estrogen-progesterone level, generalized horizontal bone loss in OPG), the case was provisionally misdiagnosed as generalized chronic periodontitis (ignoring patient’s drug history) and traditional Phase-I therapy was performed twice at six weeks interval. Gingival response was re-evaluated three months thereafter; when inflammatory condition of gingiva was improved enough but GE was not reversed (Figure 2). When the lesion was not reversed following withdrawal of the pill or by repeated non-surgical measures, incisional biopsies were performed at keratinized gingival tissues, disto-buccal to second molars of each quadrant and submitted for microscopic examination.
Figure 1.
Orthopantomogram revealing generalized alveolar bone loss.
Figure 2.
Non-reversal of gingival overgrowth at re-evaluation phase.
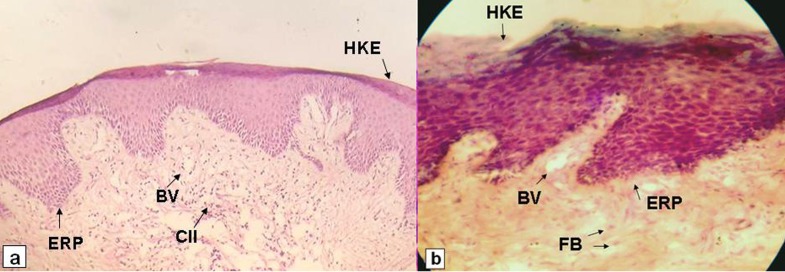
Tissue sections revealed variably thick hyper-keratinized stratified squamous epithelium and elongated, broad retepegs. The connective tissue stroma was thick, edematous but fibrous in nature, containing mature fibroblasts, mild degree of chronic inflammatory infiltrates and numerous, small blood vessels. These characteristic microscopic findings (Figure 3a, b) were very similar to GE in pregnancy or long standing pyogenic granuloma. Extensive patient’s history, clinical, radiographic and especially microscopic findings were interpreted as diagnostically consistent with OC induced periodontal endocrinopathies (i.e., attachment loss, gingival enlargement).
Figure 3.
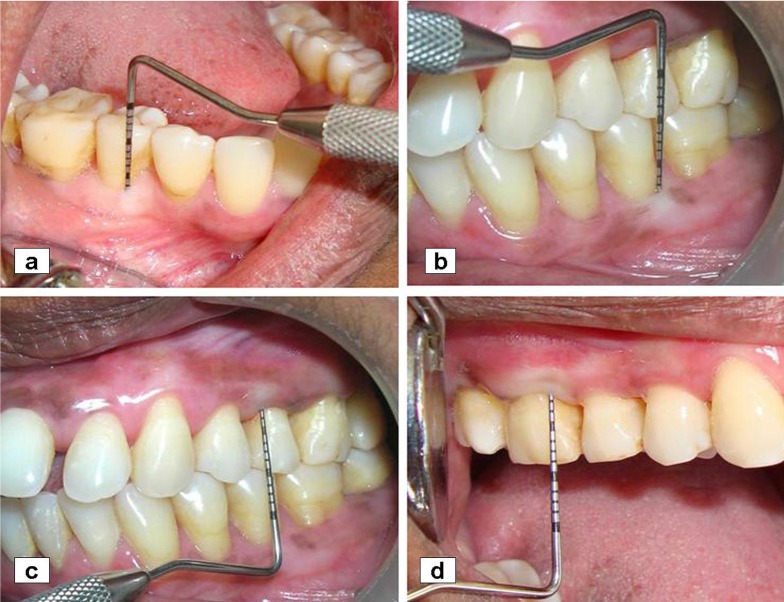
Remarkably reduced pocket depth post operatively in a) mandibular right, b) mandibular left, c) maxillary left, and d) maxillary right quadrant.
Surgical intervention was planned for correction of enlarged gingiva to prevent progressive periodontal breakdown and to maintain better access for plaque control. Each quadrant was treated according to criteria for surgical method selection [i.e., probing pocket depth (PPD), remaining AG, pattern of bone loss, furcation defects in OPG]. Periodontal surgical procedures were planned in the following manner for each quadrant: (a) Right maxillary canine to second molar - Modified widman flap surgery (i.e., PPD >4mm, AG >2mm). (b) Left maxillary canine to second molar –Apically repositioned flap (ARF) surgery with modification buccally (designed to preserve adequate width of AG), internal bevel gingivectomy palatally, along with excision of localised gingival excess over maxillary tuberosity. (c) Left mandibular lateral incisor to second molar - Similar ARF surgery (PPD ⩾5mm, CAL ⩾5mm, AG <2mm, severe GE buccally) with modification. (d) Right mandibular canine to second molar - Internal bevel gingivectomy was designed to remove moderate amount of enlarged gingiva (PPD ± 4mm, remaining AG ⩾3mm, CAL ⩽3mm) bucco-lingually.
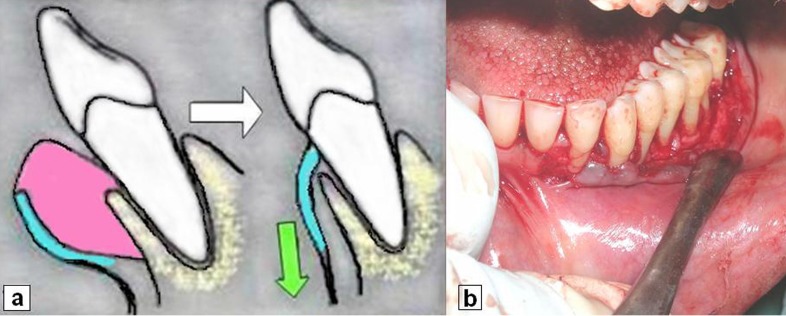
Standard surgical procedures were performed according to treatment plan.7 Basic surgical procedures were modified at second and third quadrant. In either area, split-thickness flap was prepared by sharp dissection up to the level of mucogingival junction to create a thin flap outside as well as to sacrifice entire gingival excess internally (Figure 4a, b). Then full-thickness flap was elevated apically to facilitate repositioning of flap towards mucobuccal fold. Loop electrode was used to excise localised gingival excess over left maxillary tuberosity. Involved furcations were managed by curettage and debridement alone. Histological examination of excised gingival tissue was repeated. Close monthly follow up for first postoperative (p.o) year and three monthly thereafter, were scheduled to assess possible relapse, progressive attachment loss and to provide comprehensive maintenance care.
Figure 4.
(a) Schematic illustration of the technique used for preparation of thin flap (blue area) by removing excess gingival tissues (pink area) and to displace it in apical direction (green arrow), (b) clinical photograph applying the same technique.
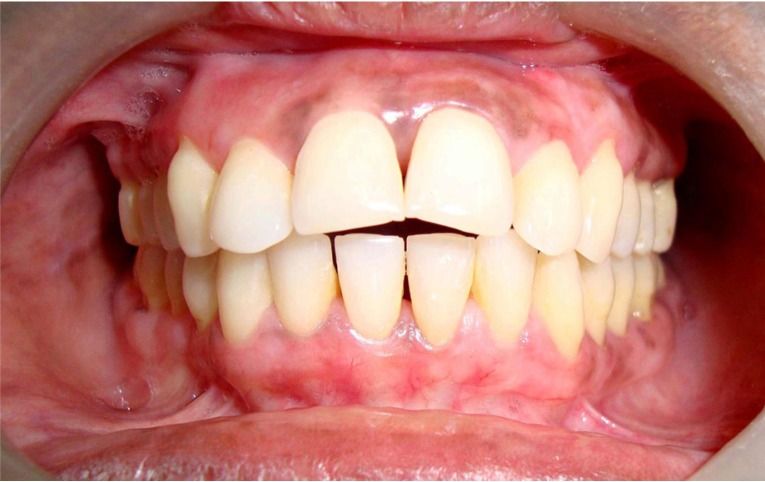
Pocket depth (Figure 5), tooth mobility and gingival enlargement were reduced remarkably (Table 1) but attachment gain was minimal. No difference was observed in the microscopic findings of the samples cross-checked. Although periodontal surgery was satisfactory but p.o plaque control and patient compliance provided excellent response to therapy. The patient has been closely followed for over 3 years and to date reveals no evidence of recurrence of GE (Figure6) and progressive attachment loss.
Figure 5.
Remarkably reduced pocket depth post operatively in a) mandibular right, b) mandibular left, c) maxillary left, and d) maxillary right quadrant.
Figure 6.
No evidence of recurrence of gingival enlargement at the end of 3 years.
DISCUSSION
Combined pill is the most popular, most efficacious OC agent and one of the most widely used classes of drugs in the world.8 Nonetheless, OCIPE are relatively scarce in recent decades, perhaps due to current low dose OC formulations, alternate withdraw/choice of contraception methods, good plaque control and NST. An understanding of the etiology of periodontal endocrinopathies is essential for the prevention and/or treatment of sex steroid hormone induced periodontal diseases. Gingival changes are related to the stimulation of specific populations of fibroblasts by estrogen, increased vascular permeability and proliferation.2 Both the sex hormones decrease gingival immune response to plaque bacteria.9 Inflamed gingival tissues are capable of metabolizing sex hormones to active metabolites at higher rate;10 thus, local irritants may exaggerate OC induced gingival changes. Therefore, the response of the periodontium in OCIPE is probably multifactorial in nature where dose, duration of pill usage, dental plaque and sex hormone-sensitive cells are the key modifying factors.2,5 Literatures reported gingival changes with the use of high-dose OC compositions (⩾50 μg/day estrogen and ⩾4mg progesterone/day) prior to the 1980s but rare with current low dose formulations.4,11 In our case, patient received low dose OC formulation over 5 years. Lynestrenol is strong synthetic progesterone, which has additional weak estrogenic effect.12 So gingival changes observed in our case are assumed to be related with additional estrogenic and strong progestational effects of the pill. The periodontium would appear to be an odd target of OCs.2 In gingival tissues, estrogen is responsible for keratinization and proliferative changes in epithelium and increased fibroblastic activity.2 Progesterone increases proliferation, dilatation, tortuosity and permeability of gingival microvasculatures, facilitates bone resorption, decreases collagen production; thus promoting tissue catabolism and delaying repair.2,13,14 Hence, estrogen and especially progesterone in OC can contribute to periodontal changes similar to pregnancy.3,5 Our reported case was in excellent agreement with previous study which demonstrated the possibility of gingival changes with low dose OC formulations.15 An interesting aspect of this case relates to the fact that the GE was not reversed either on discontinuing OC or by repeated SRP, though literatures revealed complete disappearance in most cases.6 Physiological concentrations of estradiol were found to stimulate gingival fibroblast proliferation in vitro.2 In our case, persistence of GE even after withdrawal of OC questions the role of physiologically fluctuating level of sex hormones related to menstrual cycle on presensitized gingival cells. Moreover, presence of deep pockets and GE hinder the adequacy of oral hygiene measures which may be another cause of persistence of GE.
The non-responsiveness to NST raises the suspicion of an alternate etiology. Therefore, it was necessary to determine the nature of lesion or to rule out diverse causes such as idiopathic GE, and hemopoetic malignancies by incisional biopsies of gingiva prior to surgery. Although long standing history limits the possibility of malignant involvement but clinical findings (gum bleeding, non-reversibility of GE) led us to consider it for differential diagnosis. Light microscopy revealed consistent spectrum of findings similar to that seen in pregnancy and so most likely related to OC use. In retrospect, there was initially a hint of underlying OCIPE in patient’s history. Thus, composite evidences (i.e., history, clinical-radiological-histological findings) led us to interpret the case finally as OCIPE.
Soft, vascular gingival tissues were difficult to manage during surgery but p.o healing was uneventful. Osseous resection and regenerative surgery were decided not to be performed in the presence of mobile teeth. Curettage and debridement of involved furcations alone were sufficient to maintain the function of those compromised teeth.
She remains free of recurrence to date and continues to be monitored on a regular basis. The psychological benefits of cosmetic improvement must not be underestimated. Periodontists are in a prime position to counsel the effects of OCs in oral health and to treat unhealthy periodontal conditions. This case reaffirms the fact that periodontal surgery, patient compliance and comprehensive maintenance care are essential to return to healthy periodontal status in such conditions.
REFERENCES
- 1.Mascarenhas P, Gapski R, Al-Shammari K, Wang H-L. Influence of sex hormones on the periodontium. J Clin Periodontol. 2003;30:671–681. doi: 10.1034/j.1600-051x.2003.00055.x. [DOI] [PubMed] [Google Scholar]
- 2.Mariotti A. Sex steroid hormones and cell dynamics in the periodontium. Crit Rev Oral Biol Med. 1994;5:27–53. doi: 10.1177/10454411940050010201. [DOI] [PubMed] [Google Scholar]
- 3.Klokkevold PR, Mealey BL. Influence of systemic disorders and stress on the periodontium. In: Newman MG, Takei HH, Klokkevold PR, Carranza FA, editors. Carranza’s Clinical Periodontology. 10’th Ed. St Louis, USA: Saunders; 2006. pp. 284–311. [Google Scholar]
- 4.Palmer R, Soory M. Modifying Factors. In: Lindhe J, Lang NP, Karring T, editors. Clinical Periodontology and Implant Dentistry. 5’th Ed. Oxford, USA: Blackwell Munksgaard; 2008. pp. 307–327. [Google Scholar]
- 5.Nassrawin NA, Al- Najdawi WA, Shakkoury WA. The effects of the oral contraceptive pill lo-femenal on the gingival and periodontal health. Journal of The Royal Medical Services. 2010;17(Suppl 1):7–9. [Google Scholar]
- 6.Preshaw PM, Knutsen MA, Mariotti A. Experimental gingivitis in women using oral contraceptives. J Dent Res. 2001;80:2011–2015. doi: 10.1177/00220345010800111201. [DOI] [PubMed] [Google Scholar]
- 7.Carranza FA, Takei HH. The periodontal flap. In: Newman MG, Takei HH, Klokkevold PR, Carranza FA, editors. Carranza’s Clinical Periodontology. 10’th Ed. St Louis, USA: Saunders; 2006. pp. 926–949. [Google Scholar]
- 8.Mascarenhas P, Gapski R, Al-Shammari K, Wang H-L. Influence of sex hormones on the periodontium. J Clin Periodontol. 2003;30:671–681. doi: 10.1034/j.1600-051x.2003.00055.x. [DOI] [PubMed] [Google Scholar]
- 9.Amar S, Chung KM. Influence of hormonal variation on the periodontium in women. Periodontology 2000. 1994;6:79–87. doi: 10.1111/j.1600-0757.1994.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 10.Ojanotko-Harri A. Metabolism of progesterone by healthy and inflamed human gingiva in vitro. J Steroid Biochem. 1985;23:1031–1035. doi: 10.1016/0022-4731(85)90063-9. [DOI] [PubMed] [Google Scholar]
- 11.Brown C, Ling F, Wan J. A new monophasic oral contraceptive containing drospirenone. Effect on premenstrual symptoms. J Reprod Med. 2002;47:14–22. [PubMed] [Google Scholar]
- 12.Naari empowering women/Lynestrenol. URL: http://www.naari.ch/category/index/25/181/active-pharmaceutical-ingredients/lynestrenol.html. Accessed June 14, 2010.
- 13.Otomo-Corgel J. Periodontal therapy in the female patient. In: Newman MG, Takei HH, Klokkevold PR, Carranza FA, editors. Carranza’s Clinical Periodontology. 10’th Ed. St Louis, USA: Saunders; 2006. pp. 636–649. [Google Scholar]
- 14.Raber-Durlacher JE, Van Steenbergen TJM, Van der Velden U, De Graaff J, Abraham-Inpijn L. Experimental gingivitis during pregnancy and post-partum: clinical, endocrinological and microbiological aspects. J Clin Periodontol. 1994;21:549–558. doi: 10.1111/j.1600-051x.1994.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 15.Tilakaratne A, Soory M, Ranasinghe AW, Corea SMX, Ekanayake SL, De Silva M. Effects of hormonal contraceptives on the periodontium in a population of rural Sri-Lankan women. J Clin Periodontol. 2000;27:753–757. doi: 10.1034/j.1600-051x.2000.027010753.x. [DOI] [PubMed] [Google Scholar]