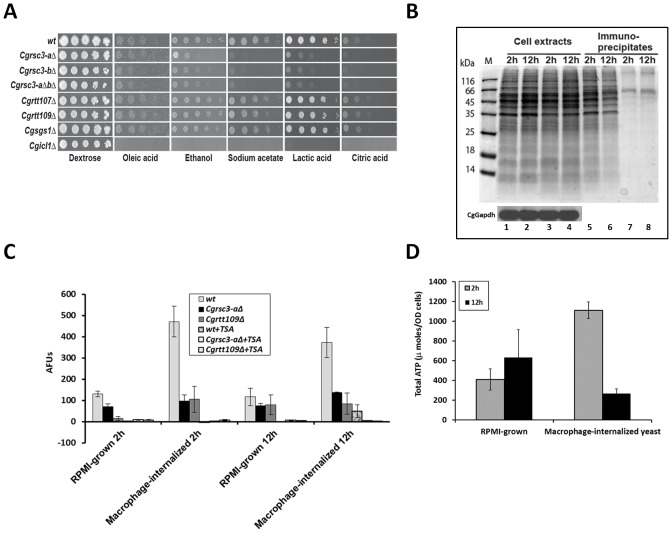
Figure 5. Macrophage-internalized C. glabrata cells display elevated lysine deacetylase activity.
(A) Serial dilution-spotting assay to assess the growth of C. glabrata strains on plates containing indicated compounds as sole carbon sources. (B) Acetylation status of cellular proteins was checked by resolving the immunoprecipitates, pulled down with anti-acetylated lysine antibody, on SDS-PAGE. Lanes 1–2, 5–6, and 3–4, 7–8 represent RPMI-growth and macrophage internalization conditions, respectively. The supernatant fraction of the cell extracts, after immuno-precipitation with anti-acetyl lysine antibody, was probed with anti-Gapdh antibody, and similar levels of CgGapdh protein in RPMI-grown and macrophage-internalized samples were observed (bottom panel). (C) Cellular lysine deacetylase activity was measured using trifluoroacetyl-lysine as a substrate. Treatment with 10 nM trichostatin A (TSA) brought the KDAC activity to basal levels thus validating the specificity of the assay. Data represent the mean of three independent analyses (± SEM). (D) Cellular ATP was extracted from wt cells with trichloroacetic acid and quantified by the luciferase activity assay using the ATP bioluminescent kit. Data normalized to total viable yeast CFU counts at indicated time points is plotted and represent mean of three independent analyses (± SEM).