Abstract
Natural killer T cells (NKT cells) represent a subset of T lymphocytes that express natural killer (NK) cell surface markers. A subset of NKT cells, termed invariant NKT cells (iNKT), express a highly restricted T cell receptor (TCR) and respond to CD1d-restricted lipid ligands. iNKT cells are now appreciated to play an important role in linking innate and adaptive immune responses and have been implicated in infectious disease, allergy, asthma, autoimmunity, and tumor surveillance. Advances in iNKT identification and purification have allowed for the detailed study of iNKT activity in both humans and mice during a variety of chronic and acute infections. Comparison of iNKT function between non-pathogenic simian immunodeficiency virus (SIV) infection models and chronic HIV-infected patients implies a role for iNKT activity in controlling immune activation. In vitro studies of influenza infection have revealed novel effector functions of iNKT cells including IL-22 production and modulation of myeloid-derived suppressor cells, but ex vivo characterization of human iNKT cells during influenza infection are lacking. Similarly, as recent evidence suggests iNKT involvement in dengue virus pathogenesis, iNKT cells may modulate responses to a number of emerging pathogens. This Review will summarize current knowledge of iNKT involvement in responses to viral infections in both human and mouse models and will identify critical gaps in knowledge and opportunities for future study. We will also highlight recent efforts to harness iNKT ligands as vaccine adjuvants capable of improving vaccination-induced cellular immune responses.
Introduction
The immune response to invading pathogens requires the successful activation of innate immunity, which informs the development of the subsequent adaptive immune response. A small subset of T lymphocytes expressing surface markers characteristic of both T cells and natural killer (NK) cells are now appreciated to form an important link between the innate and adaptive immune responses. These NKT cells can be activated in both antigen-dependent and independent manners and respond with robust Th1 and Th2 cytokine production, allowing them to exhibit remarkable functional plasticity with both pro-inflammatory and immunoregulatory characteristics. NKT cells can be grouped into several subsets (Table 1), but the most commonly described group is the Type 1 or invariant NKT (iNKT) subset, which is the focus of this Review. iNKTs are highly conserved among mouse, non-human primate (NHP) species, and humans [1]–[4] and are so named due to the expression of a highly restricted T cell receptor (TCR) repertoire. In humans and NHPs, iNKT cells are characterized by expression of a TCR comprised of Vα24-Jα18 paired with Vβ11 (reviewed in Porcelli [5]), while mouse iNKTs express Vα14-Jα18 paired with one of Vβ8.2, Vβ7, or Vβ2 [6]. The majority of iNKTs express CD161 (NK1.1 in mice) and all respond to lipid ligands through CD1d restriction. Despite the low frequency of the iNKT population in the periphery (0.01%–1% of CD3+ lymphocytes in humans), iNKT activity is now appreciated to play important roles in infectious disease, allergy, autoimmunity, and tumor surveillance. This review will focus on the current understanding and gaps in knowledge regarding iNKT function during human viral infection. A description of iNKT function during viral infection in mouse models has previously been reviewed by Diana et al. [7].
Table 1. Human and mouse CD1d-restricted NKT cell subsets [130]–[134].
| NKT Cell Subset | Mouse | Human | |
| Type I | TCR | Vα14-Jα18; Vβ8.2/7/2 | Vα24-Jα18; Vβ11 |
| Subsets | CD4+, DN | CD4+, CD8+, DN | |
| Ligand | αGalCer | αGalCer | |
| Restriction | CD1d | CD1d | |
| NK receptors | NK1.1+/− | CD161+/− | |
| Type II | TCR | Vα3.2-Jα9 or Vα8; Vβ8 | Diverse |
| Subsets | CD4+, DN | CD4+, CD8+ | |
| Ligand | Sulfatide, lysosulfatide, lysophosphatidylcholine | Sulfatide, lysosulfatide, lysophosphatidylcholine | |
| Restriction | CD1d | CD1d | |
| NK receptors | NK1.1+/− | CD161+ |
iNKT Thymic Selection and Development
Current knowledge regarding iNKT thymic selection has recently been thoroughly reviewed by Hu et al. [8]. Like conventional T cells, iNKTs develop in the thymus from CD4+CD8+ thymocytes. Expression of the iNKT TCR is selected by reactivity with CD1d-presented endogenous lipid, which directs cells to the iNKT lineage; the contribution of high-affinity ligand negative selection to iNKT development is still unclear but may also play a role [9], [10]. Signaling from both the TCR and signaling lymphocyte-activation molecule (SLAM) receptors is required for iNKT development. Maturation and proliferation of iNKT cells can occur either in the periphery or the thymus, with mature iNKT cells requiring IL-15 for maintenance [11]. Determinants of iNKT maturation are not fully understood, but were recently shown to involve microRNA-150 expression in mice [12], [13].
iNKT Activation by Ligand-Dependent and Independent Mechanisms
iNKT TCR–mediated responses are restricted by CD1d, a member of the non-polymorphic CD1 antigen presenting protein family [5], which promotes the presentation of endogenous [14] and pathogen-derived [15]–[26] lipid antigens to the TCR [27]. Although no viral-associated lipid iNKT antigens have been described, iNKT activation in the absence of a pathogen-derived lipid antigen can occur in a CD1d-dependent or independent manner (reviewed in Brigl et al. [22] and Matsuda et al. [28]). iNKT activation by antigen presenting cell (APC)-mediated lipid antigen presentation involves IL-12 production and is strongly dependent on CD40/CD40L interactions [29], with low levels of CD40L being detectible ex vivo on the surface of iNKT cells [30], [31] and intracellular, pre-formed CD40L mobilized upon activation [32]. Numerous pathogen-derived lipid antigens have now been identified from bacterial species (reviewed in [33]) and the endogenous lipid β-D-glucopyranosylceramide was recently shown to accumulate in APCs following infection and to activate mouse and human iNKTs [14]. Additionally, both gram negative and gram positive bacteria are capable of activating iNKT cells via TLR stimulation of, and IL-12/IFNα/β production by, APCs [26], [34]–[37]. This mechanism appears to require CD1d-restricted presentation of endogenous lipid. Finally, non-specific CD1d-independent iNKT activation can occur in the context of lipopolysaccharide (LPS)-induced APC production of IL-12 and IL-18 [38]. Given the lack of viral lipid antigens available for CD1d presentation, the capacity to be activated by APC cytokine production allows the iNKT subset to respond to viral infections as well as bacterial and parasitic infections. Indeed, new evidence demonstrates that weak TCR stimulation by endogenous lipids temporarily “primes” iNKT cells to rapidly respond to cytokine activation signals, emphasizing the broad, innate responsiveness of the iNKT subset during infection [39].
iNKT Subsets and Functional Capacity
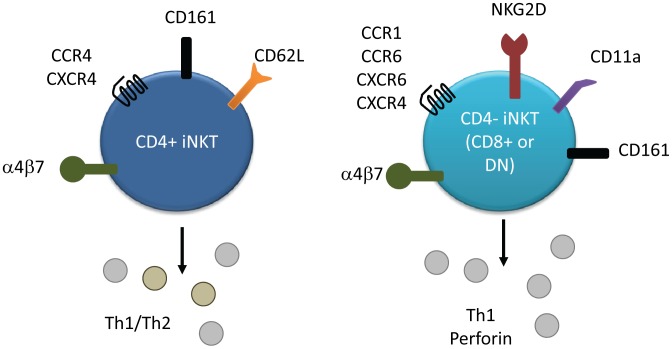
Human iNKTs express CD4 and CD8α [40], [41], allowing iNKT subsets to be defined as CD4+, CD4−CD8− (DN), or CD8+. Subset-specific differences in surface marker expression have been described (Figure 1) [30], with CD4+ iNKTs exhibiting lower expression of CCR5 but increased expression of CCR4 compared to the CD4− subset, which characteristically expresses CCR1, CCR6, CXCR6, and NKG2D (reviewed in Kim et al. [42]). All iNKTs express high levels of CXCR3 and CXCR4 and typically exhibit an effector/memory phenotype [43], [44]. The CD4− subset tends to express low levels of CD62L but higher levels of CD11a, suggesting a tissue-infiltrating phenotype, while the CD4+ subset preferentially expresses CD62L and therefore exhibits a lymph node homing phenotype [45]. CD4 and CD8 are both expressed on thymic iNKT cells, but the CD4+ subset predominates. Expansion in the periphery therefore appears to account for the increased proportion of CD8+/DN iNKTs observed outside the thymus [46]. While CD4 expression has known functional consequences during iNKT activation [47], [48], a similar functional impact for CD8 expression has not been described.
Figure 1. Surface marker and cytokine expression of human iNKT cell subsets.
Both subsets express CD161, α4β7, and high levels of CXCR4. CD4+ iNKTs preferentially express CCR4 and CD62L, and produce both Th1 and Th2 cytokines. CD4− iNKTs preferentially express chemokine receptors CCR1, CCR6, and CXCR6, as well as CD11a and NKG2D. This subset secretes predominately Th1 cytokines and more quickly secretes perforin than the CD4+ subset.
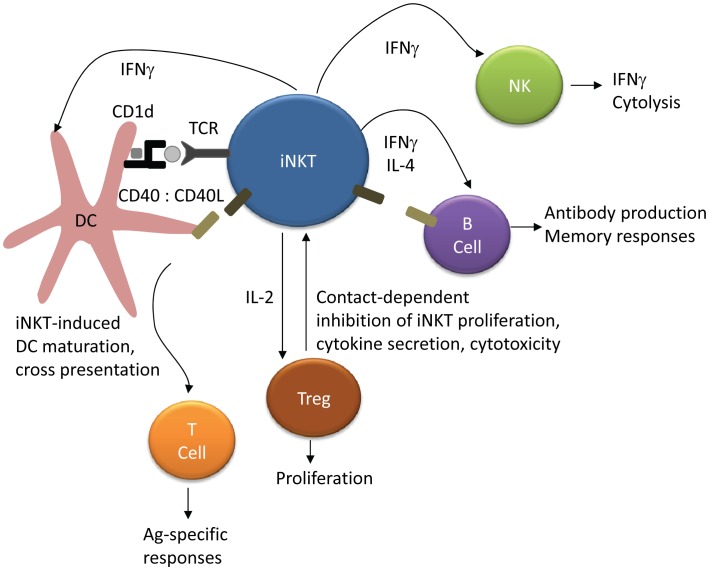
A hallmark of iNKT activation is the rapid production of a vast array of cytokines and chemokines [49], [50] including IFNγ, TNFα, TGFβ, GM-CSF, IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, IL-17, IL-21, RANTES, Eotaxin, MIP-1α, and MIP-1β (reviewed in Matsuda et al. and Tessmer et al. [28], [49]). CD4+ iNKTs produce both Th1 and Th2 cytokines, while CD4− iNKTs generally produce only Th1 cytokines [30], [51] (reviewed in Kim et al. [42]). Other iNKT effector functions include perforin/granzyme release [28], [51], [52], and Fas/FasL-mediated cytotoxicity [28], [49]. iNKTs can play an important role in the activation and regulation of multiple immune cell subsets (Figure 2), including NK, T cell, regulatory T cell, and B cell activation [53]–[55]. Stimulation of iNKT cells in conjunction with soluble T cell antigen enhances both CD4+ and CD8+ antigen-specific responses via a mechanism involving CD40 signaling [56]. Similarly, iNKT activation improves antibody titres, substitutes for CD4+ T cell help to B cells, and enhances B cell memory in mice [57].
Figure 2. iNKT regulation of NK, T cell, and B cell activation.
Presentation of lipid antigen to iNKT cells by DCs leads to iNKT activation and upregulation of CD40L. CD40–CD40L interactions and iNKT cytokine secretion promotes DC activation and maturation, which in turn leads to antigen cross-presentation and augmentation of CD4+ and CD8+ T cell responses. iNKT IFNγ secretion rapidly activates NK cells and induces further IFNγ secretion. iNKTs can substitute for CD4+ T cell help in B cell activation through CD40–CD40L interactions, and iNKT activation improves antibody titres and B cell memory responses. Finally, iNKT production of IL-2 induces regulatory T cell (Treg) proliferation, while Tregs can also inhibit iNKT proliferation and functional responses.
The functional plasticity of iNKT cells, combined with their ability to modulate activation of other immune cell subsets, suggests that they may play both a protective role in controlling viral infection and a detrimental role in enhancing viral pathogenesis. Here, we review the current understanding of the roles of iNKT cells in human viral infections, with particular focus on HIV and influenza infection (summarized in Table 2).
Table 2. Summary of iNKT studies in viral pathogenesis.
| Mouse | Human | |||
| Frequency | Function | Frequency | Function | |
| HIV | N/A | N/A | Depletion of total and CD4+ subset [45], [58]; variable recovery after HAART [58], [60], [64]–[66] | Inhibition of IFNγ, IL-4 and proliferation [61], [64], [70]; iNKT cells demonstrate anti-HIV activity [61] |
| HBV | Increase in hepatic type II NKT cells during acute hepatitis [135] | Activation enhances HBV-specific T cell responses [87]; promote IFNγ-dependent viral inhibition [85] | N/A | N/A |
| HCV | N/A | N/A | Variable depletion following infection in viremic individuals [91]–[94] | CXCR3 upregulation [94]; greater Th2 cytokine production after expansion [94] |
| HSV | N/A | Required for viral load control, protection from mortality [105], [106] | N/A | CD1d downregulation reduces iNKT activation [102] |
| Influenza | N/A | Activation promotes effective NK and CD8+ response [113]; control of viral titre [111] | N/A | Activation reduces the suppressive capacity of MDSCs, improves antigen-specific responses [110] |
HAART, highly active antiretroviral therapy; MDSC, myeloid-derived suppressor cell.
Chronic Viral Infections
Human Immunodeficiency Virus
CD4+ iNKT depletion
iNKT cell frequency is significantly reduced among HIV-1 positive individuals [45], [58], with a specific depletion of the CD4+ iNKT subset compared to the CD4− subset [45]. Longitudinal analysis of pre-seroconversion and 1 year and 5 year post-seroconversion samples demonstrated significant iNKT loss within the first year of infection, with continual declines by 5 years [58]. Expression of CCR5 and CXCR4 on CD4+ iNKTs [43], [45], [59] makes them susceptible to infection with R5-tropic, X4-tropic, and primary isolate viruses [43], [45], [59], resulting in the preferential depletion of CD4+ iNKT cells during in vitro infection [45]. The lack of change in CD4− iNKT populations during in vitro infection suggests that loss of the CD4+ subset is not largely due to CD4 downregulation. Replication of similar studies in a number of cohorts has largely confirmed these initial observations [60]–[63], although the impact of highly active antiretroviral therapy (HAART) on iNKT cell reconstitution remains controversial [58], [64]–[66] and the kinetics of iNKT reconstitution appear to be slower than that of conventional CD4+ T cells [62], [67].
Although it is now agreed that iNKT cells, particularly CD4+ iNKTs, are depleted during HIV-1 infection, less data is available to clarify the impact of this depletion on disease progression and viral pathogenesis. While it appears that the iNKT subset is involved in the host response to viral infection, it is unknown whether iNKT activation could control HIV replication and immune activation, or what role the iNKT subset might play in anti-tumor responses and prevention of opportunistic infections in immunocompromised hosts [68]. One study to date has demonstrated iNKT cell culture supernatant inhibition of HIV p24 production during in vitro CD4+ T cell infection, which was shown to be IFNγ-dependent [61]. In a study of risk factors involved in developing cancer among HIV-1 positive women, NKT cell frequency was associated with a reduced risk of cancer [69]. While further studies are required to assess the increased risk of progression or co-infection, if any, associated with iNKT decline, it is clear that both iNKT number and function are affected by HIV infection, as discussed below.
iNKT dysfunction
Even among individuals with minimal iNKT depletion during HIV-1 infection, the iNKT subset displays functional impairment [61], [64], [70]. Both CD4+ and CD4− iNKTs exhibit reduced proliferation and IFNγ, TNFα, and IL-4 secretion in response to αGalCer/IL-2/PMA stimulation [61], [64], [70], with variable restoration among HAART recipients. Increased iNKT expression of exhaustion marker programmed death (PD)-1 was reported among HIV-1 positive individuals in one study [64], but PD-1 levels did not significantly correlate with IFNγ production or proliferative capacity and PD-1 blockade did not restore iNKT function. While Moll et al. suggest that this implies an irreversibly exhausted phenotype, the expression and functional impact of other inhibitory receptors such as 2B4, Tim-3, and LAG-3 on the iNKT subset during HIV-1 infection remains unknown. As evidence now suggests that the function of Tim-3 differs between T cell and NK cell subsets [71], [72], the precise impact of exhaustion marker regulation on iNKT cells during infection must be determined. Additionally, in the Vasan et al. study, stimulations were carried out on iNKT-enriched PBMC cultures that were B cell– and CD8+ T cell–depleted [61]. Given that the unique functional properties of CD8+ iNKT cells and the ability of B cells to present lipid antigen via CD1d are now appreciated, the depletion of these subsets could influence the cytokine production of the iNKT population. More data is also required to address the dysfunction of CD8+ versus DN iNKT subsets during HIV infection, as studies to date have often failed to differentiate these subsets.
Non-human primates and SIV infection
Other clues as to the importance of iNKT activation during HIV-1 infection may come from NHP studies of SIV infection. In vivo infection of macaques with SHIVmn229 and SIVmac251 resulted in CD4+ iNKT depletion similar to human HIV-1 infection, and iNKT depletion was tightly correlated to CD4 decline [73]. Animals capable of viral control exhibited reduced CD4+ iNKT decline, and iNKT levels were inversely correlated with viral load. The similarities in iNKT depletion between HIV and SIV infection provide a model to investigate iNKT activation during natural control of infection. Unlike macaques, Sooty mangabeys (SM) control immune activation during chronic SIV infection and do not exhibit progressive immunodeficiency. SM iNKT cells are either CD8+ or CD4−CD8− and express neither CD4 nor CCR5 [74]. As a result, the iNKT subset is maintained following infection and exhibits no impairment in IFNγ production. Given the capacity of SM iNKTs to produce IFNγ, TNFα, IL-2, IL-13, and IL-10 and to degranulate in response to αGalCer stimulation, the authors speculate that iNKTs may play a role in controlling immune activation in this model of natural infection. As murine iNKT IL-4 and IL-10 production can induce regulatory T cell (Treg) development [75], the production of IL-10 by SM iNKTs is of particular interest. Additionally, iNKT-pDC cross-talk during mouse viral infection can induce naïve T cell differentiation into Tregs, suggesting another potential mechanism of iNKT-mediated Treg activation [76]. Maintenance of Tregs during SIV infection is a characteristic of natural SIV control [77], and despite the low frequency of peripheral iNKT cells, the role of iNKT activation in promoting Treg maintenance and controlling immune activation during infection should be further examined [74].
CD1d downregulation
While iNKT cells are depleted and exhausted during HIV infection, CD1d expression is also modulated by the virus itself. The HIV-1 protein Nef, responsible for the downregulation of MHC-I A and B alleles [78], also downregulates CD1d via a common tyrosine-based motif [79], [80]. This downregulation was shown in vitro to reduce NKT activation and IFNγ secretion after αGalCer stimulation [79], [80].
Hepatitis
Murine NKT cells are highly enriched in the liver (comprising 10%–30% of T cells) [81], [82], and murine models have clearly demonstrated a crucial role for NKT cell activation in mediating liver pathology in viral- and ConA-induced hepatitis [83]. While many studies have focused on αGalCer-mediated iNKT activation and autoimmune-like models of hepatitis, less is known about the role of NKT and iNKT cells in control of acute and chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection in humans. Although human iNKTs do not appear to be highly enriched in the liver compared to peripheral blood, further characterization of human hepatic iNKT subsets is required [84]. Unlike studies of stringently defined iNKT cells in HIV infection, mouse hepatitis studies include a range of NKT subsets and definitions, making it more difficult to draw comparisons from study to study.
Hepatitis B virus
Transgenic mouse models of HBV infection have suggested iNKT control of HBV replication through hepatic IFNα/β/γ induction and NK activation [85], [86]. αGalCer-activated Vα14+ iNKT cells also enhance the generation of HBV-specific cytotoxic T lymphocytes (CTLs) following HBsAg-immunization [87], suggesting a potential mechanism by which to promote viral clearance during chronic infection. Studies of NKT function in human HBV infection are currently lacking. Injection of αGalCer in a clinical trial resulted in a significant decrease in iNKT (Vα24+Vβ11+) frequency following treatment, but only one patient exhibited a sustained decrease in HBV DNA levels [88]. Other HBV literature reports only on NKT cells (defined as CD3+CD56+), a cell subset that does not necessarily overlap with the iNKT subset. One group reported a significant drop in NKT (CD3+CD56+) frequency in the first weeks after hospital admission among acute hepatitis B patients, and suggested trafficking of NKT cells to the liver as a potential explanation [89], while a study in India reported significantly increased NKT (CD3+CD56+/CD16+) frequency among acute, but not fulminant, HBV cases [90]. Further characterization of human NKT cells, including more specific delineation of iNKT/NKT subsets, during acute and chronic HBV infection will be required to understand their role in innate immune control of the virus.
Hepatitis C virus
Description of peripheral and hepatic iNKT cells during human HCV infection has been highly inconsistent. One study reported significantly lower peripheral blood iNKT (Vα24+Vβ11+) frequency among viremic compared to aviremic HCV seropositive individuals and healthy controls [91]. A similar depletion of hepatic Vα24+ iNKTs was observed among cirrhotic HCV disease patients [92]. Conversely, other studies reported no change in peripheral iNKT frequency between healthy and seropositive individuals [93], [94], nor any correlation with serum HCV RNA titre [94]. Longitudinal analysis showed no change in iNKT frequency following antiviral therapy, or differences between responders and nonresponders [93].
Functional data suggests that iNKT cells may traffic to the liver during HCV infection and acquire a fibrogenic cytokine producing profile. CXCR3 is upregulated on iNKT cells among HCV+ patients [94], possibly due to the increased hepatic levels of IP-10 and MIG during infection [95], [96]. Following expansion of iNKTs derived from HCV+ individuals, IFNγ production negatively correlated and IL-4 positively correlated with serum RNA titre, indicating a potential role for iNKTs in control of HCV replication. Interestingly, iNKTs from HCV+ patients produced more IL-13 and tended toward greater Th2 cytokine production [94]. Given that iNKT cells contribute to liver fibrosis during chronic viral hepatitis via production of IL-4 and IL-13 [97]–[100], this data supports the idea of iNKT functional modification toward a pathogenic cytokine secretion profile.
Latent/relapsing viruses
HSV. In the context of herpes virus infections, evidence is emerging to support viral interference of iNKT function. Kaposi's sarcoma-associated herpesvirus (KSHV) was the first to be shown to possess the ability to downregulate CD1d expression, an effect mediated by the viral modulation of immune recognition proteins MIR1 and MIR2 [101]. Similarly, herpes simplex virus type I (HSV-1) infection of human peripheral monocytes and immature dendritic cells results in rapid downregulation of CD1d expression via glycoprotein B and US3 [102], [103]. This downregulation results in decreased DC-mediated activation of human NKT cell lines and is thought to facilitate viral evasion of the iNKT-mediated immune response. Interestingly, HSV infection of keratinoctyes does not induce CD1d downregulation, but, through a contact-dependent mechanism, inhibits iNKT cytokine secretion and induces an anergic-like iNKT phenotype [104]. The mechanism of inhibition was not determined, but was not mediated by iNKT PD-1 or Tim-3 expression, suggesting the involvement of an additional, dominant inhibitory pathway. Given the lack of effect of PD-1 or Tim-3 blocking in restoring iNKT function during viral infection (HSV and HIV), it remains to be seen whether multiple inhibitory receptors contribute in each case. The effect of NKT cells in the early response to infection has been studied using a zosteriform model of HSV-1 infection. iNKT-deficient mice have been shown to suffer increased morbidity, enhanced spread of the virus in the nervous system, and diminished ability to clear the virus [105].
In murine models, CD1d−/− mice exhibit significantly higher HSV-1 viral load within dorsal root ganglia, larger skin lesions, and greater neuronal death than wild-type mice, indicating that efficient early viral control requires intact iNKT cells [106]. These results could not, however, be replicated by another group using a different viral strain [107], although the differences in virulence between the viruses used in these studies is worth noting [108]. Similarly, susceptibility of mice to intravaginal challenge with HSV-2 has been studied in several naïve knockout mouse strains. The NKT-deficient mice exhibited intermediate mortality and a 10-fold lower lethal dose compared to wild-type mice [109].
Acute Viral Infection
Influenza
iNKT cells in host response to influenza infection
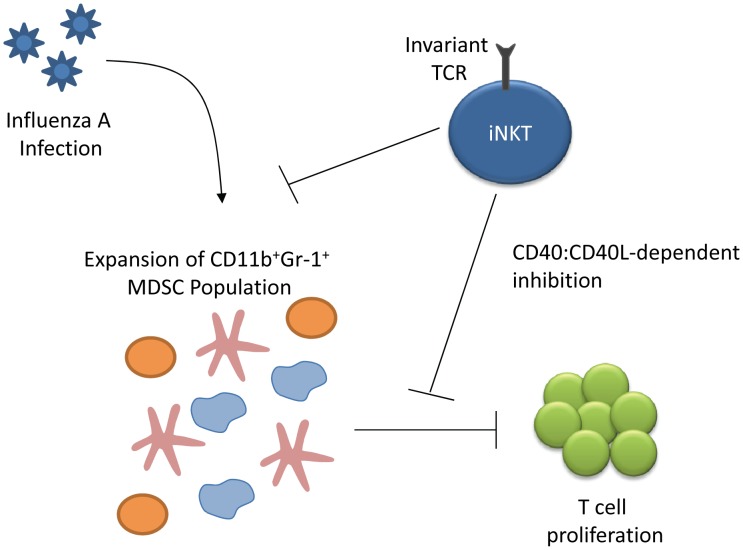
A novel role for iNKT cells in modulating immune activity was discovered when De Santo et al. reported the identification of myeloid-derived suppressor cells (MDSCs) that could inhibit influenza-specific immune responses and result in increased viral titres and mortality [110]. The group demonstrated that in both mice and humans, iNKT cells functioned to reduce the suppressive capacity of the MDSCs and improved influenza-specific responses (Figure 3). Similarly, activation of iNKT cells boosted early innate immune responses and reduced viral titre [111]. Although iNKTs have not previously been reported to produce IL-22, Paget et al. recently reported that activation of DC TLR7 and RIG-I during murine H3N2 infection results in IL-1β- and IL-23-mediated signals that induce iNKT IL-22 secretion [112]. While IL-22 production was not found to affect viral replication, it did protect epithelial cells from damage in vitro. Influenza infection of CD1d-deficient mice also suggests that iNKT-mediated IFNγ production is required for full NK and CD8+ T cell activation and antiviral activity [113], although these results are inconsistent with other studies of CD1−/− mice [114]. In a high pathogenicity model of murine influenza infection, iNKT cells were implicated in the control of infiltrating inflammatory monocytes. Activated iNKT cells were also shown to directly lyse infected monocytes in vitro [115]. Increased consistency in the virulence of strains used in challenge experiments and the genetic background of mouse strains will be required in order to conclusively determine the effects of iNKT activation during influenza infection. To our knowledge, only one study has examined iNKT frequency during human influenza infection, but did report a 20% decrease in absolute NKT counts among severe cases of pandemic H1N1 infection [116].
Figure 3. iNKT modulation of myeloid-derived suppressor cells (MDSCs) elicited during influenza A infection.
Influenza infection leads to the expansion of the MDSC population (comprised of immature dendritic cells, immature macrophages, and granulocytes), which can inhibit T cell proliferation in vivo and in vitro. iNKT cells suppress both the expansion of the MDSCs and the suppressive effect of MDSCs in a CD40-CD40L-dependent manner [110].
Use of αGalCer as a vaccine adjuvant to activate iNKT cells
The vast majority of literature on iNKT cells in influenza infection focuses on the role of αGalCer and iNKT activation as a vaccine adjuvant in mouse models. Initially, it was shown that nasal administration of αGalCer with the antigen PR8 HA (influenza virus A/PR/8/34 (PR8, H1N1)) induced high levels of systemic IgG and mucosal S-IgA Abs, high levels of IFN-γ and IL-4 both locally and systemically, and Ag-specific CTLs. These responses were associated with complete protection against an influenza viral challenge [117]. Subsequent studies documented augmented influenza antibody responses induced by co-administration of vaccine with αGalCer [57], [118]. The study by Galli et al. reported an increased influenza-specific CD4+ T cell response after co-administration of vaccine with the adjuvant. They demonstrated that the adjuvant led to activation of iNKT cells, which in turn resulted in antibody responses even in the absence of CD4+ T cells (MHC class II knockout mice), an effect not reproduced by T cell adjuvants such as alum. The authors concluded that iNKT cells can compensate for the absence of CD4+ T cell help [57].
Several lines of evidence also suggest that the stimulation of iNKT cells influences the subsequent cell mediated response to influenza. Administration of αGalCer with a high dose of an inactivated, non-replicating virus had a strong iNKT activating effect; however, this was accompanied by diminished peak CD8+ response to the immunodominant nucleoprotein epitope (NP366). Interestingly, increased NP366-specific memory CD8+ responses were demonstrated after 6 weeks in the group that received the adjuvant. Taken together, this study indicates a blunted antigen-specific effector CTL response that is followed by an enhanced CD8+ recall [119]. Similarly, injection of αGalCer during murine cytomegalovirus infection also resulted in increased CD8+ central memory cell frequency, further supporting a role for iNKT activation in antigen-specific memory responses [120].
The potential for boosting of both antibody responses and CD8+ memory by stimulation of iNKT cells is appealing in the context of providing cross-protection against emerging strains of influenza. Use of αGalCer as an adjuvant for a live attenuated NS1truncated vaccine has been shown to increase IgG, IgG1, and IgG2a antibodies as well as IFN-γ secreting CD8+ T cells, in an iNKT-dependent manner [121]. Indeed, cross-protection induced by mucosal influenza vaccine along with iNKT cell adjuvant was illustrated by high levels of nasal IgA and cross-protection against a challenge with a non-vaccine strain [122]. Similarly, Lee et al. used two αGalCer analogues with different cytokine release profiles along with inactivated influenza vaccine and were able to induce antibody responses and achieve better cellular immune responses; however, the ability to induce cross-protection was not directly studied [123]. Overall, the use of αGalCer as a vaccine adjuvant to stimulate iNKT cell activation may result in an enhanced mucosal antibody response, improved generation of CD8+ memory, and greater responses to recall antigen. αGalCer may be a particularly useful adjuvant for mucosal immunizations, as mucosal iNKT cells do not become anergic following activation, in contrast to some cases of peripheral iNKT activation [124]. Biochemical modifications of CD1d ligands to produce αGalCer analogues that elicit specific iNKT cytokine secretion profiles will further enhance the utility of iNKT activation as immunotherapy [125], [126]. The fine-tuning of this technique to induce robust memory and cross-protection against emerging influenza strains is promising, and provides a new avenue for vaccine research.
Conclusions and Future Directions
Since the identification of iNKT cells just over a decade ago, better characterization of CD4+ and CD8+ subsets and description of the growing list of roles they play in bridging innate and adaptive responses has led to appreciation of their importance in the orchestrated response to viral infections (summarized in Table 2). Perhaps most impressive is the amount of information that has been collected in the HIV field with ample evidence of the targeting of these cells by the virus and specific viral effects on CD1d expression, leading to early depletion and dysfunction of the iNKT population. Many questions remain, however, with regards to the kinetics of these changes immediately after acquisition of HIV and the true potential of antiretroviral therapy to reverse dysfunction. NHP studies may play an important role in illuminating whether iNKT cells can contribute to protection from infection at mucosal surfaces or to the control of immune activation and disease progression. Determining whether iNKT cells play a similar role in chronic HBV and HCV infections will require a focus on studies of human infection and improved consistency in the detection and definition of iNKT populations.
In contrast to the plethora of research in the context of HIV as well as other chronic and persistent infections, a paucity of data is available in the context of acute, resolving infections. The vast majority of studies are based on murine models with obvious limitations in their applicability to humans. An accumulation of excellent studies focused on the ability of adjuvants directed at activation of iNKT cells, and co-administered with influenza vaccine formulations, to lead to the generation of a robust humoral and cell mediated immunity is intriguing. Most of these studies use mouse models but hold promise by demonstrating a mechanism that may improve influenza vaccine's ability to result in long lasting CD8+ memory and potentially lead to better cross-protection against newly arising viral strains. As an appreciation of the impact of iNKT activity on viral immunity continues to increase, iNKT cells will likely be found to contribute to host defence in a number of other viral infections. CD1d downregulation appears to be a common immune evasion tactic among viruses, and has also been identified in human papillomavirus (HPV) infection [127]. Some evidence suggests the mast cell–mediated recruitment of NKT cells to sites of dengue virus infection [128] and a potentially detrimental role during pathogenesis in mouse models of infection [129]. As we better understand the mechanisms by which iNKT cells contribute to viral immunity, the therapeutic potential of modulating their activation and function will drive new research avenues.
Funding Statement
KRF is supported by the Canadian Institutes for Health Research (CIHR). JAJ is supported by the CIHR International Infectious Disease and Global Health Training Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Brossay L, Chioda M, Burdin N, Koezuka Y, Casorati G, et al. (1998) CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med 188: 1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wun KS, Borg NA, Kjer-Nielsen L, Beddoe T, Koh R, et al. (2008) A minimal binding footprint on CD1d-glycolipid is a basis for selection of the unique human NKT TCR. J Exp Med 205: 939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Couedel C, Peyrat MA, Brossay L, Koezuka Y, Porcelli SA, et al. (1998) Diverse CD1d-restricted reactivity patterns of human T cells bearing “invariant” AV24BV11 TCR. Eur J Immunol 28: 4391–4397. [DOI] [PubMed] [Google Scholar]
- 4. Kashiwase K, Kikuchi A, Ando Y, Nicol A, Porcelli SA, et al. (2003) The CD1d natural killer T-cell antigen presentation pathway is highly conserved between humans and rhesus macaques. Immunogenetics 54: 776–781. [DOI] [PubMed] [Google Scholar]
- 5. Porcelli SA, Modlin RL (1999) The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol 17: 297–329. [DOI] [PubMed] [Google Scholar]
- 6. Matsuda JL, Gapin L, Fazilleau N, Warren K, Naidenko OV, et al. (2001) Natural killer T cells reactive to a single glycolipid exhibit a highly diverse T cell receptor beta repertoire and small clone size. Proc Natl Acad Sci U S A 98: 12636–12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diana J, Lehuen A (2009) NKT cells: friend or foe during viral infections? Eur J Immunol 39: 3283–3291. [DOI] [PubMed] [Google Scholar]
- 8. Hu T, Gimferrer I, Alberola-Ila J (2011) Control of early stages in invariant natural killer T-cell development. Immunology 134: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chun T, Page MJ, Gapin L, Matsuda JL, Xu H, et al. (2003) CD1d-expressing dendritic cells but not thymic epithelial cells can mediate negative selection of NKT cells. J Exp Med 197: 907–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pellicci DG, Uldrich AP, Kyparissoudis K, Crowe NY, Brooks AG, et al. (2003) Intrathymic NKT cell development is blocked by the presence of alpha-galactosylceramide. Eur J Immunol 33: 1816–1823. [DOI] [PubMed] [Google Scholar]
- 11. Gordy LE, Bezbradica JS, Flyak AI, Spencer CT, Dunkle A, et al. (2011) IL-15 Regulates Homeostasis and Terminal Maturation of NKT Cells. J Immunol 187: 6335–6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bezman NA, Chakraborty T, Bender T, Lanier LL (2011) miR-150 regulates the development of NK and iNKT cells. J Exp Med 208: 2717–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zheng Q, Zhou L, Mi QS (2012) MicroRNA miR-150 Is Involved in Valpha14 Invariant NKT Cell Development and Function. J Immunol 188: 2118–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brennan PJ, Tatituri RV, Brigl M, Kim EY, Tuli A, et al. (2011) Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol 12: 1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burrows PD, Kronenberg M, Taniguchi M (2009) NKT cells turn ten. Nat Immunol 10: 669–671. [DOI] [PubMed] [Google Scholar]
- 16. Kawakami K, Yamamoto N, Kinjo Y, Miyagi K, Nakasone C, et al. (2003) Critical role of Valpha14+ natural killer T cells in the innate phase of host protection against Streptococcus pneumoniae infection. Eur J Immunol 33: 3322–3330. [DOI] [PubMed] [Google Scholar]
- 17. Kinjo Y, Illarionov P, Vela JL, Pei B, Girardi E, et al. (2011) Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat Immunol 12: 966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mattner J, Savage PB, Leung P, Oertelt SS, Wang V, et al. (2008) Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe 3: 304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, et al. (2006) Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol 7: 978–986. [DOI] [PubMed] [Google Scholar]
- 20. Kumar H, Belperron A, Barthold SW, Bockenstedt LK (2000) Cutting edge: CD1d deficiency impairs murine host defense against the spirochete, Borrelia burgdorferi. J Immunol 165: 4797–4801. [DOI] [PubMed] [Google Scholar]
- 21. Tupin E, Benhnia MR, Kinjo Y, Patsey R, Lena CJ, et al. (2008) NKT cells prevent chronic joint inflammation after infection with Borrelia burgdorferi. Proc Natl Acad Sci U S A 105: 19863–19868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brigl M, Brenner MB (2010) How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens. Semin Immunol 22: 79–86. [DOI] [PubMed] [Google Scholar]
- 23. Fischer K, Scotet E, Niemeyer M, Koebernick H, Zerrahn J, et al. (2004) Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc Natl Acad Sci U S A 101: 10685–10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, et al. (2005) Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434: 520–525. [DOI] [PubMed] [Google Scholar]
- 25. Wu D, Xing GW, Poles MA, Horowitz A, Kinjo Y, et al. (2005) Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc Natl Acad Sci U S A 102: 1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mattner J, Debord KL, Ismail N, Goff RD, Cantu C 3rd, et al. (2005) Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434: 525–529. [DOI] [PubMed] [Google Scholar]
- 27. Grant EP, Degano M, Rosat JP, Stenger S, Modlin RL, et al. (1999) Molecular recognition of lipid antigens by T cell receptors. J Exp Med 189: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L (2008) CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr Opin Immunol 20: 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Godfrey DI, Rossjohn J (2011) New ways to turn on NKT cells. J Exp Med 208: 1121–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee PT, Benlagha K, Teyton L, Bendelac A (2002) Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med 195: 637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bendelac A, Savage PB, Teyton L (2007) The biology of NKT cells. Annu Rev Immunol 25: 297–336. [DOI] [PubMed] [Google Scholar]
- 32. Koguchi Y, Buenafe AC, Thauland TJ, Gardell JL, Bivins-Smith ER, et al. (2012) Preformed CD40L is stored in Th1, Th2, Th17, and T follicular helper cells as well as CD48 thymocytes and invariant NKT cells but not in Treg cells. PLoS ONE 7: e31296 doi:10.1371/journal.pone.0031296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pei B, Vela JL, Zajonc D, Kronenberg M (2012) Interplay between carbohydrate and lipid in recognition of glycolipid antigens by natural killer T cells. Ann N Y Acad Sci 1253: 68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB (2003) Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol 4: 1230–1237. [DOI] [PubMed] [Google Scholar]
- 35. Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, et al. (2007) Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity 27: 597–609. [DOI] [PubMed] [Google Scholar]
- 36. Raftery MJ, Winau F, Giese T, Kaufmann SH, Schaible UE, et al. (2008) Viral danger signals control CD1d de novo synthesis and NKT cell activation. Eur J Immunol 38: 668–679. [DOI] [PubMed] [Google Scholar]
- 37. Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, et al. (2011) Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med 208: 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nagarajan NA, Kronenberg M (2007) Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol 178: 2706–2713. [DOI] [PubMed] [Google Scholar]
- 39. Wang X, Bishop KA, Hegde S, Rodenkirch LA, Pike JW, et al. (2012) Human invariant natural killer T cells acquire transient innate responsiveness via histone H4 acetylation induced by weak TCR stimulation. J Exp Med 209: 987–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takahashi T, Chiba S, Nieda M, Azuma T, Ishihara S, et al. (2002) Cutting edge: analysis of human V alpha 24+CD8+ NK T cells activated by alpha-galactosylceramide-pulsed monocyte-derived dendritic cells. J Immunol 168: 3140–3144. [DOI] [PubMed] [Google Scholar]
- 41. Ishihara S, Nieda M, Kitayama J, Osada T, Yabe T, et al. (1999) CD8(+)NKR-P1A (+)T cells preferentially accumulate in human liver. Eur J Immunol 29: 2406–2413. [DOI] [PubMed] [Google Scholar]
- 42. Kim CH, Butcher EC, Johnston B (2002) Distinct subsets of human Valpha24-invariant NKT cells: cytokine responses and chemokine receptor expression. Trends Immunol 23: 516–519. [DOI] [PubMed] [Google Scholar]
- 43. Motsinger A, Haas DW, Stanic AK, Van Kaer L, Joyce S, et al. (2002) CD1d-restricted human natural killer T cells are highly susceptible to human immunodeficiency virus 1 infection. J Exp Med 195: 869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. D'Andrea A, Goux D, De Lalla C, Koezuka Y, Montagna D, et al. (2000) Neonatal invariant Valpha24+ NKT lymphocytes are activated memory cells. Eur J Immunol 30: 1544–1550. [DOI] [PubMed] [Google Scholar]
- 45. Sandberg JK, Fast NM, Palacios EH, Fennelly G, Dobroszycki J, et al. (2002) Selective loss of innate CD4(+) V alpha 24 natural killer T cells in human immunodeficiency virus infection. J Virol 76: 7528–7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baev DV, Peng XH, Song L, Barnhart JR, Crooks GM, et al. (2004) Distinct homeostatic requirements of CD4+ and CD4− subsets of Valpha24-invariant natural killer T cells in humans. Blood 104: 4150–4156. [DOI] [PubMed] [Google Scholar]
- 47. Chen X, Wang X, Besra GS, Gumperz JE (2007) Modulation of CD1d-restricted NKT cell responses by CD4. J Leukoc Biol 82: 1455–1465. [DOI] [PubMed] [Google Scholar]
- 48. Thedrez A, de Lalla C, Allain S, Zaccagnino L, Sidobre S, et al. (2007) CD4 engagement by CD1d potentiates activation of CD4+ invariant NKT cells. Blood 110: 251–258. [DOI] [PubMed] [Google Scholar]
- 49. Tessmer MS, Fatima A, Paget C, Trottein F, Brossay L (2009) NKT cell immune responses to viral infection. Expert Opin Ther Targets 13: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chang YJ, Huang JR, Tsai YC, Hung JT, Wu D, et al. (2007) Potent immune-modulating and anticancer effects of NKT cell stimulatory glycolipids. Proc Natl Acad Sci U S A 104: 10299–10304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gumperz JE, Miyake S, Yamamura T, Brenner MB (2002) Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med 195: 625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takahashi T, Nieda M, Koezuka Y, Nicol A, Porcelli SA, et al. (2000) Analysis of human V alpha 24+ CD4+ NKT cells activated by alpha-glycosylceramide-pulsed monocyte-derived dendritic cells. J Immunol 164: 4458–4464. [DOI] [PubMed] [Google Scholar]
- 53. Carnaud C, Lee D, Donnars O, Park SH, Beavis A, et al. (1999) Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol 163: 4647–4650. [PubMed] [Google Scholar]
- 54. La Cava A, Van Kaer L, Fu Dong S (2006) CD4+CD25+ Tregs and NKT cells: regulators regulating regulators. Trends Immunol 27: 322–327. [DOI] [PubMed] [Google Scholar]
- 55. Hua J, Liang S, Ma X, Webb TJ, Potter JP, et al. (2011) The interaction between regulatory T cells and NKT cells in the liver: a CD1d bridge links innate and adaptive immunity. PLoS ONE 6: e27038 doi:10.1371/journal.pone.0027038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, et al. (2003) NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol 171: 5140–5147. [DOI] [PubMed] [Google Scholar]
- 57. Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, et al. (2007) Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci U S A 104: 3984–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van der Vliet HJ, von Blomberg BM, Hazenberg MD, Nishi N, Otto SA, et al. (2002) Selective decrease in circulating V alpha 24+V beta 11+ NKT cells during HIV type 1 infection. J Immunol 168: 1490–1495. [DOI] [PubMed] [Google Scholar]
- 59. Fleuridor R, Wilson B, Hou R, Landay A, Kessler H, et al. (2003) CD1d-restricted natural killer T cells are potent targets for human immunodeficiency virus infection. Immunology 108: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chiappini E, Betti L, Bonsignori F, Azzari C, Galli L, et al. (2010) CD4(+) and CD4(−) CD1D-restricted natural killer T cells in perinatally HIV-1 infected children receiving highly active antiretroviral therapy. Int J Immunopathol Pharmacol 23: 665–669. [DOI] [PubMed] [Google Scholar]
- 61. Vasan S, Poles MA, Horowitz A, Siladji EE, Markowitz M, et al. (2007) Function of NKT cells, potential anti-HIV effector cells, are improved by beginning HAART during acute HIV-1 infection. Int Immunol 19: 943–951. [DOI] [PubMed] [Google Scholar]
- 62. Yang OO, Wilson SB, Hultin LE, Detels R, Hultin PM, et al. (2007) Delayed reconstitution of CD4+ iNKT cells after effective HIV type 1 therapy. AIDS Res Hum Retroviruses 23: 913–922. [DOI] [PubMed] [Google Scholar]
- 63. Montoya CJ, Catano JC, Ramirez Z, Rugeles MT, Wilson SB, et al. (2008) Invariant NKT cells from HIV-1 or Mycobacterium tuberculosis-infected patients express an activated phenotype. Clin Immunol 127: 1–6. [DOI] [PubMed] [Google Scholar]
- 64. Moll M, Kuylenstierna C, Gonzalez VD, Andersson SK, Bosnjak L, et al. (2009) Severe functional impairment and elevated PD-1 expression in CD1d-restricted NKT cells retained during chronic HIV-1 infection. Eur J Immunol 39: 902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. van der Vliet HJ, van Vonderen MG, Molling JW, Bontkes HJ, Reijm M, et al. (2006) Cutting edge: Rapid recovery of NKT cells upon institution of highly active antiretroviral therapy for HIV-1 infection. J Immunol 177: 5775–5778. [DOI] [PubMed] [Google Scholar]
- 66. Moll M, Snyder-Cappione J, Spotts G, Hecht FM, Sandberg JK, et al. (2006) Expansion of CD1d-restricted NKT cells in patients with primary HIV-1 infection treated with interleukin-2. Blood 107: 3081–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li D, Xu XN (2008) NKT cells in HIV-1 infection. Cell Res 18: 817–822. [DOI] [PubMed] [Google Scholar]
- 68. Unutmaz D (2003) NKT cells and HIV infection. Microbes Infect 5: 1041–1047. [DOI] [PubMed] [Google Scholar]
- 69. Nowicki MJ, Vigen C, Mack WJ, Seaberg E, Landay A, et al. (2008) Association of cells with natural killer (NK) and NKT immunophenotype with incident cancers in HIV-infected women. AIDS Res Hum Retroviruses 24: 163–168. [DOI] [PubMed] [Google Scholar]
- 70. Mureithi MW, Cohen K, Moodley R, Poole D, Mncube Z, et al. (2011) Impairment of CD1d-restricted natural killer T cells in chronic HIV type 1 clade C infection. AIDS Res Hum Retroviruses 27: 501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gleason MK, Lenvik TR, McCullar V, Felices M, O'Brien MS, et al. (2012) Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood 119: 3064–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ndhlovu LC, Lopez-Verges S, Barbour JD, Jones RB, Jha AR, et al. (2012) Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 119: 3734–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fernandez CS, Chan AC, Kyparissoudis K, De Rose R, Godfrey DI, et al. (2009) Peripheral NKT cells in simian immunodeficiency virus-infected macaques. J Virol 83: 1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rout N, Else JG, Yue S, Connole M, Exley MA, et al. (2010) Paucity of CD4+ natural killer T (NKT) lymphocytes in sooty mangabeys is associated with lack of NKT cell depletion after SIV infection. PLoS ONE 5: e9787 doi: 10.1371/journal.pone.0009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Roelofs-Haarhuis K, Wu X, Gleichmann E (2004) Oral tolerance to nickel requires CD4+ invariant NKT cells for the infectious spread of tolerance and the induction of specific regulatory T cells. J Immunol 173: 1043–1050. [DOI] [PubMed] [Google Scholar]
- 76. Diana J, Brezar V, Beaudoin L, Dalod M, Mellor A, et al. (2011) Viral infection prevents diabetes by inducing regulatory T cells through NKT cell-plasmacytoid dendritic cell interplay. J Exp Med 208: 729–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, et al. (2009) Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog 5: e1000295 doi:10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, et al. (1999) The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10: 661–671. [DOI] [PubMed] [Google Scholar]
- 79. Chen N, McCarthy C, Drakesmith H, Li D, Cerundolo V, et al. (2006) HIV-1 down-regulates the expression of CD1d via Nef. Eur J Immunol 36: 278–286. [DOI] [PubMed] [Google Scholar]
- 80. Cho S, Knox KS, Kohli LM, He JJ, Exley MA, et al. (2005) Impaired cell surface expression of human CD1d by the formation of an HIV-1 Nef/CD1d complex. Virology 337: 242–252. [DOI] [PubMed] [Google Scholar]
- 81. Ajuebor MN (2007) Role of NKT cells in the digestive system. I. Invariant NKT cells and liver diseases: is there strength in numbers? Am J Physiol Gastrointest Liver Physiol 293: G651–656. [DOI] [PubMed] [Google Scholar]
- 82. Bendelac A, Rivera MN, Park SH, Roark JH (1997) Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol 15: 535–562. [DOI] [PubMed] [Google Scholar]
- 83. Biburger M, Tiegs G (2005) Alpha-galactosylceramide-induced liver injury in mice is mediated by TNF-alpha but independent of Kupffer cells. J Immunol 175: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 84. Exley MA, Koziel MJ (2004) To be or not to be NKT: natural killer T cells in the liver. Hepatology 40: 1033–1040. [DOI] [PubMed] [Google Scholar]
- 85. Kakimi K, Guidotti LG, Koezuka Y, Chisari FV (2000) Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med 192: 921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kakimi K, Lane TE, Chisari FV, Guidotti LG (2001) Cutting edge: Inhibition of hepatitis B virus replication by activated NK T cells does not require inflammatory cell recruitment to the liver. J Immunol 167: 6701–6705. [DOI] [PubMed] [Google Scholar]
- 87. Ito H, Ando K, Ishikawa T, Nakayama T, Taniguchi M, et al. (2008) Role of Valpha14+ NKT cells in the development of Hepatitis B virus-specific CTL: activation of Valpha14+ NKT cells promotes the breakage of CTL tolerance. Int Immunol 20: 869–879. [DOI] [PubMed] [Google Scholar]
- 88. Woltman AM, Ter Borg MJ, Binda RS, Sprengers D, von Blomberg BM, et al. (2009) Alpha-galactosylceramide in chronic hepatitis B infection: results from a randomized placebo-controlled Phase I/II trial. Antivir Ther 14: 809–818. [DOI] [PubMed] [Google Scholar]
- 89. Li J, Han Y, Jin K, Wan Y, Wang S, et al. (2011) Dynamic changes of cytotoxic T lymphocytes (CTLs), natural killer (NK) cells, and natural killer T (NKT) cells in patients with acute hepatitis B infection. Virol J 8: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tripathy AS, Das R, Chadha MS, Arankalle VA (2011) Epidemic of Hepatitis B with high mortality in India: association of fulminant disease with lack of CCL4 and natural killer T cells. J Viral Hepat 18: e415–422. [DOI] [PubMed] [Google Scholar]
- 91. Lucas M, Gadola S, Meier U, Young NT, Harcourt G, et al. (2003) Frequency and phenotype of circulating Valpha24/Vbeta11 double-positive natural killer T cells during hepatitis C virus infection. J Virol 77: 2251–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Deignan T, Curry MP, Doherty DG, Golden-Mason L, Volkov Y, et al. (2002) Decrease in hepatic CD56(+) T cells and V alpha 24(+) natural killer T cells in chronic hepatitis C viral infection. J Hepatol 37: 101–108. [DOI] [PubMed] [Google Scholar]
- 93. van der Vliet HJ, Molling JW, von Blomberg BM, Kolgen W, Stam AG, et al. (2005) Circulating Valpha24+Vbeta11+ NKT cell numbers and dendritic cell CD1d expression in hepatitis C virus infected patients. Clin Immunol 114: 183–189. [DOI] [PubMed] [Google Scholar]
- 94. Inoue M, Kanto T, Miyatake H, Itose I, Miyazaki M, et al. (2006) Enhanced ability of peripheral invariant natural killer T cells to produce IL-13 in chronic hepatitis C virus infection. J Hepatol 45: 190–196. [DOI] [PubMed] [Google Scholar]
- 95. Harvey CE, Post JJ, Palladinetti P, Freeman AJ, Ffrench RA, et al. (2003) Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J Leukoc Biol 74: 360–369. [DOI] [PubMed] [Google Scholar]
- 96. Apolinario A, Majano PL, Alvarez-Perez E, Saez A, Lozano C, et al. (2002) Increased expression of T cell chemokines and their receptors in chronic hepatitis C: relationship with the histological activity of liver disease. Am J Gastroenterol 97: 2861–2870. [DOI] [PubMed] [Google Scholar]
- 97. de Lalla C, Galli G, Aldrighetti L, Romeo R, Mariani M, et al. (2004) Production of profibrotic cytokines by invariant NKT cells characterizes cirrhosis progression in chronic viral hepatitis. J Immunol 173: 1417–1425. [DOI] [PubMed] [Google Scholar]
- 98. Wang H, Park O, Gao B (2011) NKT cells in liver fibrosis: Controversies or complexities. J Hepatol 55: 1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ishikawa S, Ikejima K, Yamagata H, Aoyama T, Kon K, et al. (2011) CD1d-restricted natural killer T cells contribute to hepatic inflammation and fibrogenesis in mice. J Hepatol 54: 1195–1204. [DOI] [PubMed] [Google Scholar]
- 100. Jin Z, Sun R, Wei H, Gao X, Chen Y, et al. (2011) Accelerated liver fibrosis in hepatitis B virus transgenic mice: involvement of natural killer T cells. Hepatology 53: 219–229. [DOI] [PubMed] [Google Scholar]
- 101. Sanchez DJ, Gumperz JE, Ganem D (2005) Regulation of CD1d expression and function by a herpesvirus infection. J Clin Invest 115: 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yuan W, Dasgupta A, Cresswell P (2006) Herpes simplex virus evades natural killer T cell recognition by suppressing CD1d recycling. Nat Immunol 7: 835–842. [DOI] [PubMed] [Google Scholar]
- 103. Rao P, Pham HT, Kulkarni A, Yang Y, Liu X, et al. (2011) Herpes simplex virus 1 glycoprotein B and US3 collaborate to inhibit CD1d antigen presentation and NKT cell function. J Virol 85: 8093–8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bosnjak L, Sahlstrom P, Paquin-Proulx D, Leeansyah E, Moll M, et al. (2012) Contact-Dependent Interference with Invariant NKT Cell Activation by Herpes Simplex Virus-Infected Cells. J Immunol 188: 6216–6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Grubor-Bauk B, Simmons A, Mayrhofer G, Speck PG (2003) Impaired clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant V alpha 14-J alpha 281 TCR. J Immunol 170: 1430–1434. [DOI] [PubMed] [Google Scholar]
- 106. Grubor-Bauk B, Arthur JL, Mayrhofer G (2008) Importance of NKT cells in resistance to herpes simplex virus, fate of virus-infected neurons, and level of latency in mice. J Virol 82: 11073–11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Cornish AL, Keating R, Kyparissoudis K, Smyth MJ, Carbone FR, et al. (2006) NKT cells are not critical for HSV-1 disease resolution. Immunol Cell Biol 84: 13–19. [DOI] [PubMed] [Google Scholar]
- 108. Kulkarni RR, Haeryfar SM, Sharif S (2010) The invariant NKT cell subset in anti-viral defenses: a dark horse in anti-influenza immunity? Journal of leukocyte biology 88: 635–643. [DOI] [PubMed] [Google Scholar]
- 109. Ashkar AA, Rosenthal KL (2003) Interleukin-15 and natural killer and NKT cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J Virol 77: 10168–10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. De Santo C, Salio M, Masri SH, Lee LY, Dong T, et al. (2008) Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest 118: 4036–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ho LP, Denney L, Luhn K, Teoh D, Clelland C, et al. (2008) Activation of invariant NKT cells enhances the innate immune response and improves the disease course in influenza A virus infection. Eur J Immunol 38: 1913–1922. [DOI] [PubMed] [Google Scholar]
- 112. Paget C, Ivanov S, Fontaine J, Renneson J, Blanc F, et al. (2012) Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damage. J Biol Chem 287: 8816–8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ishikawa H, Tanaka K, Kutsukake E, Fukui T, Sasaki H, et al. (2010) IFN-gamma production downstream of NKT cell activation in mice infected with influenza virus enhances the cytolytic activities of both NK cells and viral antigen-specific CD8+ T cells. Virology 407: 325–332. [DOI] [PubMed] [Google Scholar]
- 114. Benton KA, Misplon JA, Lo CY, Brutkiewicz RR, Prasad SA, et al. (2001) Heterosubtypic immunity to influenza A virus in mice lacking IgA, all Ig, NKT cells, or gamma delta T cells. J Immunol 166: 7437–7445. [DOI] [PubMed] [Google Scholar]
- 115. Kok WL, Denney L, Benam K, Cole S, Clelland C, et al. (2011) Pivotal Advance: Invariant NKT cells reduce accumulation of inflammatory monocytes in the lungs and decrease immune-pathology during severe influenza A virus infection. J Leukoc Biol 91: 357–368. [DOI] [PubMed] [Google Scholar]
- 116. Chen WW, Xie YX, Zhang YH, Feng YQ, Li BA, et al. (2010) [Changes and analysis of peripheral white blood cells and lymphocyte subsets for patients with pandemic influenza A virus (H1N1) infection]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 24: 331–333. [PubMed] [Google Scholar]
- 117. Ko SY, Ko HJ, Chang WS, Park SH, Kweon MN, et al. (2005) alpha-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol 175: 3309–3317. [DOI] [PubMed] [Google Scholar]
- 118. Youn HJ, Ko SY, Lee KA, Ko HJ, Lee YS, et al. (2007) A single intranasal immunization with inactivated influenza virus and alpha-galactosylceramide induces long-term protective immunity without redirecting antigen to the central nervous system. Vaccine 25: 5189–5198. [DOI] [PubMed] [Google Scholar]
- 119. Guillonneau C, Mintern JD, Hubert FX, Hurt AC, Besra GS, et al. (2009) Combined NKT cell activation and influenza virus vaccination boosts memory CTL generation and protective immunity. Proc Natl Acad Sci U S A 106: 3330–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Reilly EC, Thompson EA, Aspeslagh S, Wands JR, Elewaut D, et al. (2012) Activated iNKT cells promote memory CD8(+) T cell differentiation during viral infection. PLoS ONE 7: e37991 doi:10.1371/journal.pone.0037991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kopecky-Bromberg SA, Fraser KA, Pica N, Carnero E, Moran TM, et al. (2009) Alpha-C-galactosylceramide as an adjuvant for a live attenuated influenza virus vaccine. Vaccine 27: 3766–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kamijuku H, Nagata Y, Jiang X, Ichinohe T, Tashiro T, et al. (2008) Mechanism of NKT cell activation by intranasal coadministration of alpha-galactosylceramide, which can induce cross-protection against influenza viruses. Mucosal Immunol 1: 208–218. [DOI] [PubMed] [Google Scholar]
- 123. Lee YS, Lee KA, Lee JY, Kang MH, Song YC, et al. (2011) An alpha-GalCer analogue with branched acyl chain enhances protective immune responses in a nasal influenza vaccine. Vaccine 29: 417–425. [DOI] [PubMed] [Google Scholar]
- 124. Courtney AN, Thapa P, Singh S, Wishahy AM, Zhou D, et al. (2011) Intranasal but not intravenous delivery of the adjuvant alpha-galactosylceramide permits repeated stimulation of natural killer T cells in the lung. Eur J Immunol 41: 3312–3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wojno J, Jukes JP, Ghadbane H, Shepherd D, Besra GS, et al. (2012) Amide Analogs of CD1d Agonists Modulate iNKT cell-Mediated Cytokine Production. ACS Chem Biol 7: 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kerzerho J, Yu ED, Barra CM, Alari-Pahisa E, Girardi E, et al. (2012) Structural and functional characterization of a novel nonglycosidic type I NKT agonist with immunomodulatory properties. J Immunol 188: 2254–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Miura S, Kawana K, Schust DJ, Fujii T, Yokoyama T, et al. (2010) CD1d, a sentinel molecule bridging innate and adaptive immunity, is downregulated by the human papillomavirus (HPV) E5 protein: a possible mechanism for immune evasion by HPV. J Virol 84: 11614–11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. St John AL, Rathore AP, Yap H, Ng ML, Metcalfe DD, et al. (2011) Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT-cell recruitment and viral clearance. Proc Natl Acad Sci U S A 108: 9190–9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Renneson J, Guabiraba R, Maillet I, Marques RE, Ivanov S, et al. (2011) A detrimental role for invariant natural killer T cells in the pathogenesis of experimental dengue virus infection. Am J Pathol 179: 1872–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Exley MA, Tahir SM, Cheng O, Shaulov A, Joyce R, et al. (2001) A major fraction of human bone marrow lymphocytes are Th2-like CD1d-reactive T cells that can suppress mixed lymphocyte responses. J Immunol 167: 5531–5534. [DOI] [PubMed] [Google Scholar]
- 131. Kronenberg M, Gapin L (2002) The unconventional lifestyle of NKT cells. Nat Rev Immunol 2: 557–568. [DOI] [PubMed] [Google Scholar]
- 132. Kronenberg M (2005) Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol 23: 877–900. [DOI] [PubMed] [Google Scholar]
- 133. Godfrey DI, Stankovic S, Baxter AG (2010) Raising the NKT cell family. Nat Immunol 11: 197–206. [DOI] [PubMed] [Google Scholar]
- 134. Moody DB, Zajonc DM, Wilson IA (2005) Anatomy of CD1-lipid antigen complexes. Nat Rev Immunol 5: 387–399. [DOI] [PubMed] [Google Scholar]
- 135. Baron JL, Gardiner L, Nishimura S, Shinkai K, Locksley R, et al. (2002) Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity 16: 583–594. [DOI] [PubMed] [Google Scholar]