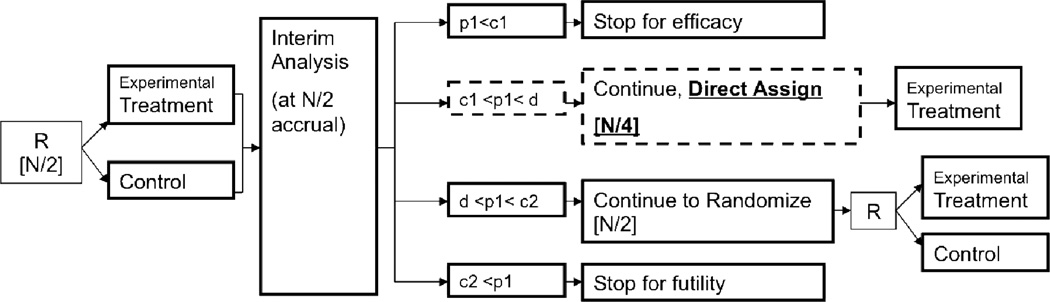
Figure 1.
Design schematic (among M+ patients only). Square brackets [] indicate number of patients enrolled at the given stage. R = randomize; N = total number of patients allocated at start of trial; p1 = p-value based on Stage I patient data; c1, c2, and d are O’Brien-Fleming stopping boundaries (d is the boundary for concluding overall trial efficacy, and is also used to decide between adopting the direct assignment option versus continuing with randomization in Stage II).